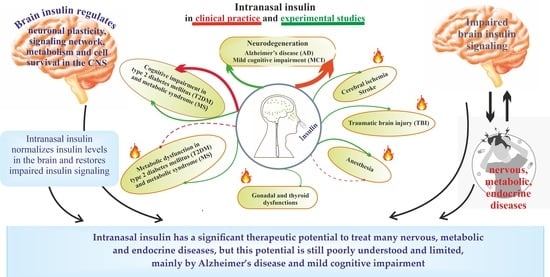
Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium
Abstract
:1. Introduction
2. Insulin and Insulin Signaling System in the Brain and Intranasal Delivery of Insulin to the Brain
2.1. Sources of Insulin in the Brain
2.2. Insulin Signaling System
2.3. The Intranasal Route of Insulin Delivery
3. Intranasal Insulin and Brain Ischemia
4. Intranasal Insulin and Brain Injury
5. Intranasal Insulin and Diabetes Mellitus
5.1. Clinical Studies
5.2. Experimental Studies
6. Intranasal Insulin and Anesthesia
6.1. Experimental Studies
6.2. Clinical Study
7. Intranasal Insulin and the Gonadal and Thyroid Systems
7.1. Gonadal Axis
7.2. Thyroidal Axis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FOXO | forkhead box family |
| GSK3β | glycogen synthase kinase-3β |
| HIF-1α | hypoxia-induced factor-1α |
| ICP | intranasally administered proinsulin C-peptide |
| IGF-1 | insulin-like growth factor-1 |
| INI | intranasally administered insulin |
| INSR | insulin receptor |
| IR | insulin resistance |
| IRS | insulin receptor substrates |
| JNK1 | c-Jun N-terminal kinase-1 |
| LPO | lipid peroxidation |
| MAPK | mitogen-activated protein kinase |
| mitoBKCa | mitochondrial ATP-sensitive large-conductance Ca2+-activated potassium channels |
| MS | metabolic syndrome |
| mTORC complex | the mammalian target of rapamycin complex |
| p21kip | a cyclin-dependent kinase inhibitor 1 |
| PDK1/2 | 3-phosphatidylinositol-dependent protein kinases of types 1 and 2 |
| PGC1α | peroxisome proliferator-activated receptor γ coactivator 1-α |
| PHLPP | PH domain and Leucine-rich repeat Protein Phosphatases |
| PI-3,4,5-P3 | phosphatidylinositol-3,4,5-triphosphate |
| PI3K | phosphatidylinositol-3-kinase |
| pNCD | postoperative neurocognitive disorder |
| POD | postoperative delirium |
| PP-1 | protein phosphatase-1 |
| PTP1B | protein phosphotyrosine phosphatase 1B |
| SHIP2 | type 2 SH2-containing inositol-5’-phosphatase |
| SREBP1 | sterol regulatory element-binding transcription factor 1 |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TBI | traumatic brain injury |
| TC-PTP | T-cell protein phosphotyrosine phosphatase |
| TSC1/2 | tuberous sclerosis proteins 1 (hamartin) and 2 (tuberin) |
References
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef]
- Havrankova, J.; Schmechel, D.; Roth, J.; Brownstein, M. Identification of insulin in rat brain. Proc. Natl. Acad. Sci. USA 1978, 75, 5737–5741. [Google Scholar] [CrossRef]
- De la Monte, S.M. Type 3 diabetes is sporadic Alzheimer′s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Xiao, M.; Chang, L.; Yan, L.J. Role of insulin resistance in Alzheimer’s disease. Metab. Brain Dis. 2015, 30, 839–851. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Tong, M.; Wands, J.R. The 20-Year Voyage Aboard the Journal of Alzheimer’s Disease: Docking at ‘Type 3 Diabetes’, Environmental/Exposure Factors, Pathogenic Mechanisms, and Potential Treatments. J. Alzheimers Dis. 2018, 62, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M. The Full Spectrum of Alzheimer’s Disease Is Rooted in Metabolic Derangements That Drive Type 3 Diabetes. Adv. Exp. Med. Biol. 2019, 1128, 45–83. [Google Scholar] [CrossRef]
- Eraky, S.M.; Ramadan, N.M.; Abo El-Magd, N.F. Ameliorative effects of bromelain on aluminum-induced Alzheimer’s disease in rats through modulation of TXNIP pathway. Int. J. Biol. Macromol. 2022, 227, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- Ardanaz, C.G.; Ramírez, M.J.; Solas, M. Brain Metabolic Alterations in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3785. [Google Scholar] [CrossRef]
- González, A.; Calfío, C.; Churruca, M.; Maccioni, R.B. Glucose metabolism and AD: Evidence for a potential diabetes type 3. Alzheimers Res. Ther. 2022, 14, 56. [Google Scholar] [CrossRef]
- Neumann, K.F.; Rojo, L.; Navarrete, L.P.; Farías, G.; Reyes, P.; Maccioni, R.B. Insulin resistance and Alzheimer’s disease: Molecular links & clinical implications. Curr. Alzheimer Res. 2008, 5, 438–447. [Google Scholar] [CrossRef]
- Frisardi, V.; Solfrizzi, V.; Seripa, D.; Capurso, C.; Santamato, A.; Sancarlo, D.; Vendemiale, G.; Pilotto, A.; Panza, F. Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 399–417. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Sun, H.; Wang, H.; Peng, W.; Zhou, Z.; Wang, H.; Pi, C.; Shi, Y.; He, X. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Huerta, M.; González-Usigli, H.A.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; Mireles-Ramírez, M.A.; Torres-Mendoza, B.M.; Moreno-Cih, R.I.; Velázquez-Brizuela, I.E. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef]
- Fan, L.W.; Carter, K.; Bhatt, A.; Pang, Y. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019, 14, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 2621. [Google Scholar] [CrossRef]
- Patel, D.; Patel, B.; Wairkar, S. Intranasal delivery of biotechnology-based therapeutics. Drug Discov. Today 2022, 27, 103371. [Google Scholar] [CrossRef]
- Sharma, M.; Waghela, S.; Mhatre, R.; Saraogi, G.K. A Recent Update on Intranasal Delivery of High Molecular Weight Proteins, Peptides, and Hormones. Curr. Pharm. Des. 2021, 27, 4279–4299. [Google Scholar] [CrossRef]
- Bose, M.; Farias Quipildor, G.; Ehrlich, M.E.; Salton, S.R. Intranasal Peptide Therapeutics: A Promising Avenue for Overcoming the Challenges of Traditional CNS Drug Development. Cells 2022, 11, 3629. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.; Maeng, H.J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Frey, W.H., 2nd; Baker, L.D.; Cholerton, B.; Keeling, M.L.; Belongia, D.A.; Fishel, M.A.; Plymate, S.R.; Schellenberg, G.D.; et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 2006, 27, 451–458. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Novak, P.; Pimentel Maldonado, D.A.; Novak, V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: A double-blinded placebo-controlled pilot study. PLoS ONE 2019, 14, e0214364. [Google Scholar] [CrossRef]
- Novak, V.; Mantzoros, C.S.; Novak, P.; McGlinchey, R.; Dai, W.; Lioutas, V.; Buss, S.; Fortier, C.B.; Khan, F.; Aponte Becerra, L.; et al. MemAID: Memory advancement with intranasal insulin vs. placebo in type 2 diabetes and control participants: A randomized clinical trial. J. Neurol. 2022, 269, 4817–4835. [Google Scholar] [CrossRef]
- Benedict, C.; Frey, W.H., 2nd; Schiöth, H.B.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp. Gerontol. 2011, 46, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Ribarič, S. The Rationale for Insulin Therapy in Alzheimer’s Disease. Molecules 2016, 21, 689. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Kalaitzidis, G.; Malli, A.; Kalaitzoglou, D.; Myserlis, P.G.; Lioutas, V.A. Intranasal insulin in Alzheimer’s dementia or mild cognitive impairment: A systematic review. J. Neurol. 2018, 265, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L.; Yang, G.; Hu, Q. Current Therapeutic Strategy in the Nasal Delivery of Insulin: Recent Advances and Future Directions. Curr. Pharm. Biotechnol. 2018, 19, 400–415. [Google Scholar] [CrossRef]
- Muñoz-Jiménez, M.; Zaarkti, A.; García-Arnés, J.A.; García-Casares, N. Antidiabetic Drugs in Alzheimer’s Disease and Mild Cognitive Impairment: A Systematic Review. Dement. Geriatr. Cogn. Disord. 2020, 49, 423–434. [Google Scholar] [CrossRef]
- Gaddam, M.; Singh, A.; Jain, N.; Avanthika, C.; Jhaveri, S.; De la Hoz, I.; Sanka, S.; Goli, S.R. A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, 13, e17219. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal insulin. J. Neuroendocrinol. 2021, 33, e12934. [Google Scholar] [CrossRef]
- Kellar, D.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal Insulin Reduces White Matter Hyperintensity Progression in Association with Improvements in Cognition and CSF Biomarker Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2021, 8, 240–248. [Google Scholar] [CrossRef]
- Miziak, B.; Błaszczyk, B.; Czuczwar, S.J. Some Candidate Drugs for Pharmacotherapy of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 458. [Google Scholar] [CrossRef]
- Long, C.; Han, X.; Yang, Y.; Li, T.; Zhou, Q.; Chen, Q. Efficacy of intranasal insulin in improving cognition in mild cognitive impairment or dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 2022, 14, 963933. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Hatke, A.; Schultes, B.; Fehm, H.L.; Born, J.; Kern, W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 2004, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Hallschmid, M.; Schmitz, K.; Schultes, B.; Ratter, F.; Fehm, H.L.; Born, J.; Kern, W. Intranasal insulin improves memory in humans: Superiority of insulin aspart. Neuropsychopharmacology 2007, 32, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Kern, W.; Schultes, B.; Born, J.; Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 2008, 93, 1339–1344. [Google Scholar] [CrossRef]
- Kupila, A.; Sipilä, J.; Keskinen, P.; Simell, T.; Knip, M.; Pulkki, K.; Simell, O. Intranasally administered insulin intended for prevention of type 1 diabetes--a safety study in healthy adults. Diabetes Metab. Res. Rev. 2003, 19, 415–420. [Google Scholar] [CrossRef]
- Schmid, V.; Kullmann, S.; Gfrörer, W.; Hund, V.; Hallschmid, M.; Lipp, H.P.; Häring, H.U.; Preissl, H.; Fritsche, A.; Heni, M. Safety of intranasal human insulin: A review. Diabetes Obes. Metab. 2018, 20, 1563–1577. [Google Scholar] [CrossRef]
- Gwizdala, K.L.; Ferguson, D.P.; Kovan, J.; Novak, V.; Pontifex, M.B. Placebo controlled phase II clinical trial: Safety and efficacy of combining intranasal insulin & acute exercise. Metab. Brain Dis. 2021, 36, 1289–1303. [Google Scholar] [CrossRef]
- Aponte Becerra, L.; Galindo Mendez, B.; Khan, F.; Lioutas, V.; Novak, P.; Mantzoros, C.S.; Ngo, L.H.; Novak, V. Safety of Intranasal Insulin in Type 2 Diabetes on Systemic Insulin: A Double-Blinded Placebo-Controlled Sub-Study of Memaid Trial. Arch. Diabetes Obes. 2022, 4, 403–415. [Google Scholar]
- Schneider, E.; Spetter, M.S.; Martin, E.; Sapey, E.; Yip, K.P.; Manolopoulos, K.N.; Tahrani, A.A.; Thomas, J.M.; Lee, M.; Hallschmid, M.; et al. The effect of intranasal insulin on appetite and mood in women with and without obesity: An experimental medicine study. Int. J. Obes. 2022, 46, 1319–1327. [Google Scholar] [CrossRef]
- Devaskar, S.U.; Giddings, S.J.; Rajakumar, P.A.; Carnaghi, L.R.; Menon, R.K.; Zahm, D.S. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J. Biol. Chem. 1994, 269, 8445–8454. [Google Scholar] [CrossRef]
- Schechter, R.; Whitmire, J.; Wheet, G.S.; Beju, D.; Jackson, K.W.; Harlow, R.; Gavin, J.R., III. Immunohistochemical and in situ hybridization study of an insulin-like substance in fetal neuron cell cultures. Brain Res. 1994, 636, 9–27. [Google Scholar] [CrossRef]
- Gerozissis, K. Brain insulin: Regulation, mechanisms of action and functions. Cell. Mol. Neurobiol. 2003, 23, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gerozissis, K. Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur. J. Pharmacol. 2008, 585, 38–49. [Google Scholar] [CrossRef]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Banks, W.A. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front. Neurosci. 2019, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Sakaguchi, M.; Lockhart, S.M.; Cai, W.; Li, M.E.; Homan, E.P.; Rask-Madsen, C.; Kahn, C.R. Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. Proc. Natl. Acad. Sci. USA 2017, 114, E8478–E8487. [Google Scholar] [CrossRef]
- Yu, Y.; Kastin, A.J.; Pan, W. Reciprocal interactions of insulin and insulin-like growth factor I in receptor-mediated transport across the blood-brain barrier. Endocrinology 2006, 147, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Jaspan, J.B.; Kastin, A.J. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides 1997, 18, 1577–1584. [Google Scholar] [CrossRef]
- Banks, W.A.; Dohgu, S.; Lynch, J.L.; Fleegal-DeMotta, M.A.; Erickson, M.A.; Nakaoke, R.; Vo, T.Q. Nitric oxide isoenzymes regulate lipopolysaccharide-enhanced insulin transport across the blood-brain barrier. Endocrinology 2008, 149, 1514–1523. [Google Scholar] [CrossRef]
- Urayama, A.; Banks, W.A. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology 2008, 149, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- May, A.A.; Bedel, N.D.; Shen, L.; Woods, S.C.; Liu, M. Estrogen and insulin transport through the blood-brain barrier. Physiol. Behav. 2016, 163, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, A.A.; Liu, M.; Woods, S.C.; Begg, D.P. CCK increases the transport of insulin into the brain. Physiol. Behav. 2016, 165, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Lam, V.; Brook, E.; Giles, C.; Fimognari, N.; Mooranian, A.; Al-Salami, H.; Coulson, S.H.; Nesbit, M.; Mamo, J.C.L. Blood-Brain Barrier Dysfunction Precedes Cognitive Decline and Neurodegeneration in Diabetic Insulin Resistant Mouse Model: An Implication for Causal Link. Front. Aging Neurosci. 2017, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Banks, W.A. Neuroimmune Axes of the Blood-Brain Barriers and Blood-Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol. Rev. 2018, 70, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 2018, 12, 930. [Google Scholar] [CrossRef] [PubMed]
- Beddows, C.A.; Dodd, G.T. Insulin on the brain: The role of central insulin signalling in energy and glucose homeostasis. J. Neuroendocrinol. 2021, 33, e12947. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Villaseñor, R.; Kuennecke, B.; Ozmen, L.; Ammann, M.; Kugler, C.; Grüninger, F.; Loetscher, H.; Freskgård, P.O.; Collin, L. Region-specific permeability of the blood-brain barrier upon pericyte loss. J. Cereb. Blood Flow Metab. 2017, 37, 3683–3694. [Google Scholar] [CrossRef]
- Mäe, M.A.; He, L.; Nordling, S.; Vazquez-Liebanas, E.; Nahar, K.; Jung, B.; Li, X.; Tan, B.C.; Chin Foo, J.; Cazenave-Gassiot, A.; et al. Single-Cell Analysis of Blood-Brain Barrier Response to Pericyte Loss. Circ. Res. 2021, 128, e46–e62. [Google Scholar] [CrossRef]
- McKinley, M.J.; Denton, D.A.; Ryan, P.J.; Yao, S.T.; Stefanidis, A.; Oldfield, B.J. From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger. J. Neuroendocrinol. 2019, 31, e12689. [Google Scholar] [CrossRef]
- Mullier, A.; Bouret, S.G.; Prevot, V.; Dehouck, B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 2010, 518, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Porniece Kumar, M.; Cremer, A.L.; Klemm, P.; Steuernagel, L.; Sundaram, S.; Jais, A.; Hausen, A.C.; Tao, J.; Secher, A.; Pedersen, T.Å.; et al. Insulin signalling in tanycytes gates hypothalamic insulin uptake and regulation of AgRP neuron activity. Nat. Metab. 2021, 3, 1662–1679. [Google Scholar] [CrossRef] [PubMed]
- Langlet, F.; Levin, B.E.; Luquet, S.; Mazzone, M.; Messina, A.; Dunn-Meynell, A.A.; Balland, E.; Lacombe, A.; Mazur, D.; Carmeliet, P.; et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell. Metab. 2013, 17, 607–617. [Google Scholar] [CrossRef]
- Prevot, V.; Langlet, F.; Dehouck, B. Flipping the tanycyte switch: How circulating signals gain direct access to the metabolic brain. Aging 2013, 5, 332–334. [Google Scholar] [CrossRef]
- Morita-Takemura, S.; Wanaka, A. Blood-to-brain communication in the hypothalamus for energy intake regulation. Neurochem. Int. 2019, 28, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Bernstein, H.G.; Hahn, H.J.; Ziegler, M.; Rummelfanger, H. Insulin immunohistochemistry of rodent CNS: Apparent species differences but good correlation with radioimmunological data. Histochemistry 1981, 71, 609–616. [Google Scholar] [CrossRef]
- Dorn, A.; Bernstein, H.G.; Rinne, A.; Hahn, H.J.; Ziegler, M. Insulin-like immunoreactivity in the human brain—A preliminary report. Histochemistry 1982, 74, 293–300. [Google Scholar] [CrossRef]
- Baskin, D.G.; Brewitt, B.; Davidson, D.A.; Corp, E.; Paquette, T.; Figlewicz, D.P.; Lewellen, T.K.; Graham, M.K.; Woods, S.G.; Dorsa, D.M. Quantitative autoradiographic evidence for insulin receptors in the choroid plexus of the rat brain. Diabetes 1986, 35, 246–249. [Google Scholar] [CrossRef]
- Hill, J.M.; Lesniak, M.A.; Pert, C.B.; Roth, J. Autoradiographic localization of insulin receptors in rat brain: Prominence in olfactory and limbic areas. Neuroscience 1986, 17, 1127–1138. [Google Scholar] [CrossRef]
- Werther, G.A.; Hogg, A.; Oldfield, B.J.; McKinley, M.J.; Figdor, R.; Allen, A.M.; Mendelsohn, F.A. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 1987, 121, 1562–1570. [Google Scholar] [CrossRef]
- Unger, J.; McNeill, T.H.; Moxley, R.T., 3rd; White, M.; Moss, A.; Livingston, J.N. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 1989, 31, 143–157. [Google Scholar] [CrossRef]
- Marks, J.L.; Porte, D., Jr.; Stahl, W.L.; Baskin, D.G. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 1990, 127, 3234–3236. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, P.; Osman, S.; Glaser, M.; Knickmeier, M.; Ferrannini, E.; Pike, V.W.; Camici, P.G.; Law, M.P. In vivo imaging of insulin receptors by PET: Preclinical evaluation of iodine-125 and iodine-124 labelled human insulin. Nucl. Med. Biol. 2002, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Chen, H.; Quon, M.J.; Alkon, D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004, 490, 71–81. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Gruber, T.; García-Cáceres, C. Insulin action on astrocytes: From energy homeostasis to behaviour. J. Neuroendocrinol. 2021, 33, e12953. [Google Scholar] [CrossRef]
- Moller, D.E.; Yokota, A.; Caro, J.F.; Flier, J.S. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol. Endocrinol. 1989, 3, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Bell, G.I. Alternative splicing of human insulin receptor messenger RNA. Biochem. Biophys. Res. Commun. 1989, 159, 312–316. [Google Scholar] [CrossRef]
- Mosthaf, L.; Grako, K.; Dull, T.J.; Coussens, L.; Ullrich, A.; McClain, D.A. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990, 9, 2409–2413. [Google Scholar] [CrossRef]
- Seino, S.; Seino, M.; Nishi, S.; Bell, G.I. Structure of the human insulin receptor gene and characterization of its promoter. Proc. Natl. Acad. Sci. USA 1989, 86, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431. [Google Scholar] [CrossRef]
- Escribano, O.; Beneit, N.; Rubio-Longás, C.; López-Pastor, A.R.; Gómez-Hernández, A. The Role of Insulin Receptor Isoforms in Diabetes and Its Metabolic and Vascular Complications. J. Diabetes Res. 2017, 2017, 1403206. [Google Scholar] [CrossRef] [Green Version]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Drakas, R.; Tu, X.; Baserga, R. Control of cell size through phosphorylation of upstream binding factor 1 by nuclear phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 9272–9276. [Google Scholar] [CrossRef] [PubMed]
- De Meyts, P. The insulin receptor: A prototype for dimeric, allosteric membrane receptors? Trends Biochem. Sci. 2008, 33, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Frasca, F.; Pandini, G.; Sciacca, L.; Vigneri, R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009, 30, 586–623. [Google Scholar] [CrossRef]
- White, M.F.; Kahn, C.R. Insulin action at a molecular level—100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef] [PubMed]
- Razzini, G.; Ingrosso, A.; Brancaccio, A.; Sciacchitano, S.; Esposito, D.L.; Falasca, M. Different subcellular localization and phosphoinositides binding of insulin receptor substrate protein pleckstrin homology domains. Mol. Endocrinol. 2000, 14, 823–836. [Google Scholar] [CrossRef]
- Sadagurski, M.; Dong, X.C.; Myers, M.G., Jr.; White, M.F. Irs2 and Irs4 synergize in non-LepRb neurons to control energy balance and glucose homeostasis. Mol. Metab. 2013, 3, 55–63. [Google Scholar] [CrossRef]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Levenga, J.; Wong, H.; Milstead, R.A.; Keller, B.N.; LaPlante, L.E.; Hoeffer, C.A. AKT isoforms have distinct hippocampal expression and roles in synaptic plasticity. eLife 2017, 6, e30640. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef]
- Brown, A.K.; Webb, A.E. Regulation of FOXO Factors in Mammalian Cells. Curr. Top. Dev. Biol. 2018, 127, 165–192. [Google Scholar] [CrossRef]
- Salcedo-Tello, P.; Ortiz-Matamoros, A.; Arias, C. GSK3 Function in the Brain during Development, Neuronal Plasticity, and Neurodegeneration. Int. J. Alzheimers Dis. 2011, 2011, 189728. [Google Scholar] [CrossRef]
- Duda, P.; Wiśniewski, J.; Wójtowicz, T.; Wójcicka, O.; Jaśkiewicz, M.; Drulis-Fajdasz, D.; Rakus, D.; McCubrey, J.A.; Gizak, A. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 2018, 22, 833–848. [Google Scholar] [CrossRef]
- Schubert, M.; Gautam, D.; Surjo, D.; Ueki, K.; Baudler, S.; Schubert, D.; Kondo, T.; Alber, J.; Galldiks, N.; Küstermann, E.; et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 2004, 101, 3100–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.W.; Donato, J., Jr.; Berglund, E.D.; Choi, Y.H.; Kohno, D.; Elias, C.F.; Depinho, R.A.; Elmquist, J.K. FOXO1 in the ventromedial hypothalamus regulates energy balance. J. Clin. Invest. 2012, 122, 2578–2589. [Google Scholar] [CrossRef]
- Ren, H.; Plum-Morschel, L.; Gutierrez-Juarez, R.; Lu, T.Y.; Kim-Muller, J.Y.; Heinrich, G.; Wardlaw, S.L.; Silver, R.; Accili, D. Blunted refeeding response and increased locomotor activity in mice lacking FoxO1 in synapsin-Cre-expressing neurons. Diabetes 2013, 62, 3373–3383. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Oli, V.; Gupta, R.; Kumar, P. FOXO and related transcription factors binding elements in the regulation of neurodegenerative disorders. J. Chem. Neuroanat. 2021, 116, 102012. [Google Scholar] [CrossRef]
- Ghasemi, R.; Haeri, A.; Dargahi, L.; Mohamed, Z.; Ahmadiani, A. Insulin in the brain: Sources, localization and functions. Mol. Neurobiol. 2013, 47, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Proud, C.G. The mTOR pathway in the control of protein synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Stoica, L.; Zhu, P.J.; Huang, W.; Zhou, H.; Kozma, S.C.; Costa-Mattioli, M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA 2011, 108, 3791–3796. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]
- Bakke, J.; Haj, F.G. Protein-tyrosine phosphatase 1B substrates and metabolic regulation. Semin. Cell Dev. Biol. 2015, 37, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Dodd, G.T.; Tiganis, T. Protein Tyrosine Phosphatases in Hypothalamic Insulin and Leptin Signaling. Trends Pharmacol. Sci. 2015, 36, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Dodd, G.T.; Xirouchaki, C.E.; Eramo, M.; Mitchell, C.A.; Andrews, Z.B.; Henry, B.A.; Cowley, M.A.; Tiganis, T. Intranasal Targeting of Hypothalamic PTP1B and TCPTP Reinstates Leptin and Insulin Sensitivity and Promotes Weight Loss in Obesity. Cell Rep. 2019, 28, 2905–2922.e5. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Nogueiras, R.; Sabio, G. Brain JNK and metabolic disease. Diabetologia 2021, 64, 265–274. [Google Scholar] [CrossRef]
- Busquets, O.; Espinosa-Jiménez, T.; Ettcheto, M.; Olloquequi, J.; Bulló, M.; Carro, E.; Cantero, J.L.; Casadesús, G.; Folch, J.; Verdaguer, E.; et al. JNK1 and JNK3: Divergent functions in hippocampal metabolic-cognitive function. Mol. Med. 2022, 28, 48. [Google Scholar] [CrossRef]
- Accardi, G.; Virruso, C.; Balistreri, C.R.; Emanuele, F.; Licastro, F.; Monastero, R.; Porcellini, E.; Vasto, S.; Verga, S.; Caruso, C.; et al. SHIP2: A "new" insulin pathway target for aging research. Rejuvenation Res. 2014, 17, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Taghibiglou, C. Elevated nuclear phosphatase and tensin homolog (PTEN) and altered insulin signaling in substantia nigral region of patients with Parkinson’s disease. Neurosci. Lett. 2018, 666, 139–143. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, L.; Jiang, L.; Hou, L.; He, L.; Zhang, C. PTEN nuclear translocation enhances neuronal injury after hypoxia-ischemia via modulation of the nuclear factor-κB signaling pathway. Aging 2021, 13, 16165–16177. [Google Scholar] [CrossRef]
- Javadpour, P.; Dargahi, L.; Ahmadiani, A.; Ghasemi, R. To be or not to be: PP2A as a dual player in CNS functions, its role in neurodegeneration, and its interaction with brain insulin signaling. Cell. Mol. Life Sci. 2019, 76, 2277–2297. [Google Scholar] [CrossRef]
- Frey, W.H., II. Neurologic Agents for Nasal Administration to the Brain. PCT International Patent WO 91/07947, 13 June 1991. [Google Scholar]
- Frey, W.H., II. Method of Administering Neurologic Agents to the Brain. U.S. Patent 5,624,898, 29 April 1997. [Google Scholar]
- Frey, W.H., II. Method for Administering Insulin to the Brain. U.S. Patent 6,313,093 B1, 6 November 2001. [Google Scholar]
- Nedelcovych, M.T.; Gadiano, A.J.; Wu, Y.; Manning, A.A.; Thomas, A.G.; Khuder, S.S.; Yoo, S.W.; Xu, J.; McArthur, J.C.; Haughey, N.J.; et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem. Neurosci. 2018, 9, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Maday, S.; Twelvetrees, A.E.; Moughamian, A.J.; Holzbaur, E.L. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014, 84, 292–309. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., II. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Salameh, T.S.; Bullock, K.M.; Hujoel, I.A.; Niehoff, M.L.; Wolden-Hanson, T.; Kim, J.; Morley, J.E.; Farr, S.A.; Banks, W.A. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. J. Alzheimers Dis. 2015, 47, 715–728. [Google Scholar] [CrossRef]
- Chudoba, C.; Kleinridders, A. 271-LB: Intranasal Insulin Treatment Causes Sex-Specific Differences in Metabolism and Behavior in Mice. Diabetes 2019, 68 (Suppl. 1), 271-LB. [Google Scholar] [CrossRef]
- Jauch-Chara, K.; Friedrich, A.; Rezmer, M.; Melchert, U.H.; Scholand-Engler, H.G.; Hallschmid, M.; Oltmanns, K.M. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 2012, 61, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.C.; Stote, R.M.; Breedt, H.J.; O’Brien, J.; Buckley, B. Pharmacokinetics and pharmacodynamics of intranasal insulin administered to healthy subjects in escalating doses. Diabetes Technol. Ther. 2005, 7, 124–130. [Google Scholar] [CrossRef]
- Leker, R.R.; Shohami, E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: Neuroprotective opportunities. Brain Res. Brain Res. Rev. 2002, 39, 55–73. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.B.; Meng, L.; Gelb, A.W.; Lee, R.; Huang, W.Q. Cerebral ischemia during surgery: An overview. J. Biomed. Res. 2016, 30, 83–87. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Maddahi, A.; Edvinsson, L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J. Neuroinflamm. 2010, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.; Lee, S.Y.; Manzanero, S.; Chunduri, P.; Sobey, C.G.; Arumugam, T.V. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev. 2013, 12, 941–966. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Emmrich, J.V.; Fricker, M.; Mander, P.K.; Théry, C.; Brown, G.C. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, E4098–E4107. [Google Scholar] [CrossRef]
- Dharmasaroja, P.A. Fluid Intake Related to Brain Edema in Acute Middle Cerebral Artery Infarction. Transl. Stroke Res. 2016, 7, 49–53. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. American Heart Association/American Stroke Association. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline from the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Pathak, P.; Gerstein, H.C. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: A systematic overview. Stroke 2001, 32, 2426–2432. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Shi, H. Hyperglycemia as a Risk Factor of Ischemic Stroke. J. Drug Metab. Toxicol. 2013, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Lioutas, V.A.; Lambadiari, V.; Paraskevas, G.P.; Voumvourakis, K.; Tsivgoulis, G. Glycemia management in acute ischemic stroke: Current concepts and novel therapeutic targets. Postgrad. Med. 2019, 131, 423–437. [Google Scholar] [CrossRef]
- Middleton, S.; McElduff, P.; Ward, J.; Grimshaw, J.M.; Dale, S.; D’Este, C.; Drury, P.; Griffiths, R.; Cheung, N.W.; Quinn, C.; et al. QASC Trialists Group. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): A cluster randomised controlled trial. Lancet 2011, 378, 1699–1706. [Google Scholar] [CrossRef]
- Passero, S.; Ciacci, G.; Ulivelli, M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology 2003, 61, 1351–1356. [Google Scholar] [CrossRef]
- Kim, Y.; Han, M.H.; Kim, C.H.; Kim, J.M.; Cheong, J.H.; Ryu, J.I. Increased Short-Term Mortality in Patients with Spontaneous Intracerebral Hemorrhage and its Association with Admission Glucose Levels and Leukocytosis. World Neurosurg. 2017, 98, 503–511. [Google Scholar] [CrossRef]
- Almdal, T.; Scharling, H.; Jensen, J.S.; Vestergaard, H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: A population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 2004, 164, 1422–1426. [Google Scholar] [CrossRef]
- Banerjee, C.; Moon, Y.P.; Paik, M.C.; Rundek, T.; Mora-McLaughlin, C.; Vieira, J.R.; Sacco, R.L.; Elkind, M.S. Duration of diabetes and risk of ischemic stroke: The Northern Manhattan Study. Stroke 2012, 43, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Mitsios, J.P.; Ekinci, E.I.; Mitsios, G.P.; Churilov, L.; Thijs, V. Relationship Between Glycated Hemoglobin and Stroke Risk: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e007858. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, W.; Wang, Y. Prediabetes and Outcome of Ischemic Stroke or Transient Ischemic Attack: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Inzucchi, S.E.; Viscoli, C.M.; Brass, L.M.; Bravata, D.M.; Shulman, G.I.; McVeety, J.C.; Horwitz, R.I. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology 2003, 60, 1447–1451. [Google Scholar] [CrossRef]
- Hishinuma, A.; Majima, M.; Kurabayashi, H. Insulin resistance in patients with stroke is related to visceral fat obesity and adipocytokines. J. Stroke Cerebrovasc. Dis. 2008, 17, 175–180. [Google Scholar] [CrossRef]
- Voll, C.L.; Auer, R.N. The effect of postischemic blood glucose levels on ischemic brain damage in the rat. Ann. Neurol. 1988, 24, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Voll, C.L.; Auer, R.N. Insulin attenuates ischemic brain damage independent of its hypoglycemic effect. J. Cereb. Blood Flow Metab. 1991, 11, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Voll, C.L.; Whishaw, I.Q.; Auer, R.N. Postischemic insulin reduces spatial learning deficit following transient forebrain ischemia in rats. Stroke 1989, 20, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.G.; Tranmer, B.I.; Auer, R.N. Insulin reduction of cerebral infarction due to transient focal ischemia. J. Neurosurg. 1995, 82, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Guyot, L.L.; Diaz, F.G.; O’Regan, M.H.; Ren, J.; Phillis, J.W. The effect of intravenous insulin on accumulation of excitotoxic and other amino acids in the ischemic rat cerebral cortex. Neurosci. Lett. 2000, 288, 61–65. [Google Scholar] [CrossRef]
- Fukuoka, S.; Yeh, H.; Mandybur, T.I.; Tew, J.M., Jr. Effect of insulin on acute experimental cerebral ischemia in gerbils. Stroke 1989, 20, 396–399. [Google Scholar] [CrossRef]
- Meden, P.; Andersen, M.; Overgaard, K.; Rasmussen, R.S.; Boysen, G. The effects of early insulin treatment combined with thrombolysis in rat embolic stroke. Neurol. Res. 2002, 24, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Lu, Y.J.; Huang, J.P.; Wu, Y.T.; Day, Y.J.; Hung, L.M. The essential role of endothelial nitric oxide synthase activation in insulin-mediated neuroprotection against ischemic stroke in diabetes. J. Vasc. Surg. 2014, 59, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Pinard, E.; Roussel, S.; Seylaz, J. Insulin protects brain tissue against focal ischemia in rats. Neurosci. Lett. 1992, 144, 121–123. [Google Scholar] [CrossRef]
- Fanne, R.A.; Nassar, T.; Heyman, S.N.; Hijazi, N.; Higazi, A.A. Insulin and glucagon share the same mechanism of neuroprotection in diabetic rats: Role of glutamate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R668–R673. [Google Scholar] [CrossRef]
- Guan, J.; Williams, C.; Gunning, M.; Mallard, C.; Gluckman, P. The effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult rats. J. Cereb. Blood Flow Metab. 1993, 13, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.Z.; Auer, R.N. Intraventricular administration of insulin and IGF-1 in transient forebrain ischemia. J. Cereb. Blood Flow Metab. 1994, 14, 237–242. [Google Scholar] [CrossRef]
- Russo, V.; Candeloro, P.; Malara, N.; Perozziello, G.; Iannone, M.; Scicchitano, M.; Mollace, R.; Musolino, V.; Gliozzi, M.; Carresi, C.; et al. Key Role of Cytochrome C for Apoptosis Detection Using Raman Microimaging in an Animal Model of Brain Ischemia with Insulin Treatment. Appl. Spectrosc. 2019, 73, 1208–1217. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Kumar, R.; Sullivan, J.M.; Krause, G.S. Insulin blocks cytochrome c release in the reperfused brain through PI3-K signaling and by promoting Bax/Bcl-XL binding. J. Neurochem. 2008, 106, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Kumar, R.; Murariu-Dobrin, A.C.; Page, A.B.; Krause, G.S.; Sullivan, J.M. Insulin activates the PI3K-Akt survival pathway in vulnerable neurons following global brain ischemia. Neurol. Res. 2009, 31, 947–958. [Google Scholar] [CrossRef]
- Duarte, A.I.; Santos, P.; Oliveira, C.R.; Santos, M.S.; Rego, A.C. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim. Biophys. Acta 2008, 1783, 994–1002. [Google Scholar] [CrossRef]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in central nervous system: More than just a peripheral hormone. J. Aging Res. 2012, 2012, 384017. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.K.; Hester, R.L. Role of nitric oxide, adenosine, and ATP-sensitive potassium channels in insulin-induced vasodilation. Hypertension 1996, 28, 202–208. [Google Scholar] [CrossRef]
- Hung, L.M.; Huang, J.P.; Liao, J.M.; Yang, M.H.; Li, D.E.; Day, Y.J.; Huang, S.S. Insulin renders diabetic rats resistant to acute ischemic stroke by arresting nitric oxide reaction with superoxide to form peroxynitrite. J. Biomed. Sci. 2014, 21, 92. [Google Scholar] [CrossRef]
- Hughes, T.M.; Craft, S. The role of insulin in the vascular contributions to age-related dementia. Biochim. Biophys. Acta 2016, 1862, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Yavuz, S. Metabolic actions of angiotensin II and insulin: A microvascular endothelial balancing act. Mol. Cell. Endocrinol. 2013, 378, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Akintola, A.A.; van Opstal, A.M.; Westendorp, R.G.; Postmus, I.; van der Grond, J.; van Heemst, D. Effect of intranasally administered insulin on cerebral blood flow and perfusion; a randomized experiment in young and older adults. Aging 2017, 9, 790–802. [Google Scholar] [CrossRef]
- Lioutas, V.A.; Alfaro-Martinez, F.; Bedoya, F.; Chung, C.C.; Pimentel, D.A.; Novak, V. Intranasal Insulin and Insulin-Like Growth Factor 1 as Neuroprotectants in Acute Ischemic Stroke. Transl. Stroke Res. 2015, 6, 264–275. [Google Scholar] [CrossRef]
- Zorina, I.I.; Zakharova, I.O.; Bayunova, L.V.; Avrova, N.F. Insulin Administration Prevents Accumulation of Conjugated Dienes and Trienes and Inactivation of Na+, K+-ATPase in the Rat Cerebral Cortex during Two-Vessel Forebrain Ischemia and Reperfusion. J. Evol. Biochem. Physiol. 2018, 54, 246–249. [Google Scholar] [CrossRef]
- Zorina, I.I.; Galkina, O.V.; Bayunova, L.V.; Zakharova, I.O. Effect of Insulin on Lipid Peroxidation and Glutathione Levels in a Two-Vessel Occlusion Model of Rat Forebrain Ischemia Followed by Reperfusion. J. Evol. Biochem. Physiol. 2019, 55, 333–335. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Bayunova, L.V.; Zorina, I.I.; Sokolova, T.V.; Shpakov, A.O.; Avrova., N.F. Insulin and α-Tocopherol Enhance the Protective Effect of Each Other on Brain Cortical Neurons under Oxidative Stress Conditions and in Rat Two-Vessel Forebrain Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 11768. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.O.; Bayunova, L.V.; Zorina, I.I.; Shpakov, A.O.; Avrova, N.F. Insulin and Brain Gangliosides Prevent Metabolic Disorders Caused by Activation of Free Radical Reactions after Two-Vessel Ischemia–Reperfusion Injury to the Rat Forebrain. J. Evol. Biochem. Physiol. 2022, 58, 279–291. [Google Scholar] [CrossRef]
- Xu, L.B.; Huang, H.D.; Zhao, M.; Zhu, G.C.; Xu, Z. Intranasal Insulin Treatment Attenuates Metabolic Distress and Early Brain Injury After Subarachnoid Hemorrhage in Mice. Neurocrit. Care 2021, 34, 154–166. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Yang, J.; Tu, R.; Zhang, X.; He, W.W.; Hou, C.Y.; Wang, X.M.; Yu, J.M.; Jiang, G.H. Intranasal insulin ameliorates neurological impairment after intracerebral hemorrhage in mice. Neural Regen. Res. 2022, 17, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Fawcett, J.R.; Thorne, R.G.; DeFor, T.A.; Frey, W.H., II. Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J. Neurol. Sci. 2001, 187, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Fawcett, J.R.; Thorne, R.G.; Frey, W.H., II. Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci. Lett. 2001, 308, 91–94. [Google Scholar] [CrossRef]
- Liu, X.F.; Fawcett, J.R.; Hanson, L.R.; Frey, W.H., II. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J. Stroke Cerebrovasc. Dis. 2004, 13, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.; Kohli, S.; Sprague, S.M.; Scranton, R.A.; Lipton, S.A.; Parra, A.; Jimenez, D.F.; Digicaylioglu, M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J. Neurosurg. 2009, 111, 164–170. [Google Scholar] [CrossRef]
- Cai, Z.; Fan, L.W.; Lin, S.; Pang, Y.; Rhodes, P.G. Intranasal administration of insulin-like growth factor-1 protects against lipopolysaccharide-induced injury in the developing rat brain. Neuroscience 2011, 194, 195–207. [Google Scholar] [CrossRef]
- Lin, S.; Rhodes, P.G.; Cai, Z. Whole body hypothermia broadens the therapeutic window of intranasally administered IGF-1 in a neonatal rat model of cerebral hypoxia-ischemia. Brain Res. 2011, 1385, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gu, X.; Wei, Z.Z.; Wu, A.; Liu, X.; Wei, L. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp. Neurol. 2021, 337, 113542. [Google Scholar] [CrossRef] [PubMed]
- Iravanpour, F.; Dargahi, L.; Rezaei, M.; Haghani, M.; Heidari, R.; Valian, N.; Ahmadiani, A. Intranasal insulin improves mitochondrial function and attenuates motor deficits in a rat 6-OHDA model of Parkinson’s disease. CNS Neurosci. Ther. 2021, 27, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kelschenbach, J.; Borjabad, A.; Hadas, E.; He, H.; Potash, M.J.; Nedelcovych, M.T.; Rais, R.; Haughey, N.J.; McArthur, J.C.; et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 2019, 33, 973–984. [Google Scholar] [CrossRef]
- Duarte, A.I.; Santos, M.S.; Oliveira, C.R.; Rego, A.C. Insulin neuroprotection against oxidative stress in cortical neurons--involvement of uric acid and glutathione antioxidant defenses. Free Radic. Biol. Med. 2005, 39, 876–889. [Google Scholar] [CrossRef]
- Sharp, F.R.; Bernaudin, M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004, 5, 437–448. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bogush, I.V.; Berstein, L.M.; Shpakov, A.O. The influence of intranasal insulin on hypothalamic-pituitary-thyroid axis in normal and diabetic rats. Horm. Metab. Res. 2015, 47, 916–924. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bondareva, V.M.; Perminova, A.A.; Shpakov, A.O. C-peptide and insulin during combined intranasal administration improve the metabolic parameters and activity of the adenylate cyclase system in the hypothalamus, myocardium, and epididymal fat of rats with type 2 diabetes. Cell Tiss. Biol. 2019, 13, 228–236. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bondareva, V.M.; Shpakov, A.O. Regulatory effects of intranasal C-peptide and insulin on thyroid and androgenic status of male rats with moderate type 1 diabetes mellitus. J. Evol. Biochem. Physiol. 2019, 55, 493–496. [Google Scholar] [CrossRef]
- Sukhov, I.B.; Derkach, K.V.; Chistyakova, O.V.; Bondareva, V.M.; Shpakov, A.O. Functional state of hypothalamic signaling systems in rats with type 2 diabetes mellitus treated with intranasal insulin. J. Evol. Biochem. Physiol. 2016, 52, 204–216. [Google Scholar] [CrossRef]
- Derkach, K.V.; Ivantsov, A.O.; Chistyakova, O.V.; Sukhov, I.B.; Buzanakov, D.M.; Kulikova, A.A.; Shpakov, A.O. Intranasal insulin restores metabolic parameters and insulin sensitivity in rats with metabolic syndrome. Bull. Exp. Biol. Med. 2017, 163, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.; Underhill, S.M.; Goldberg, M.P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr. Res. 2001, 50, 553–562. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R.; Fawcett, J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001, 24, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fan, L.W.; Rhodes, P.G.; Cai, Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp. Neurol. 2009, 217, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Matsumoto, M.; Kitagawa, K.; Matsushita, K.; Ohtsuki, T.; Mabuchi, T.; Colman, D.R.; Kamada, T.; Yanagihara, T. Ischemic damage and subsequent proliferation of oligodendrocytes in focal cerebral ischemia. Neuroscience 1997, 77, 849–861. [Google Scholar] [CrossRef]
- Mabuchi, T.; Kitagawa, K.; Ohtsuki, T.; Kuwabara, K.; Yagita, Y.; Yanagihara, T.; Hori, M.; Matsumoto, M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 2000, 31, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.; Christensen, T.; Lehrmann, E.; Diemer, N.H.; Finsen, B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp. Brain Res. 2001, 138, 384–392. [Google Scholar] [CrossRef]
- Wegner, M. Transcriptional control in myelinating glia: Flavors and spices. Glia 2000, 31, 1–14. [Google Scholar] [CrossRef]
- Tanaka, K.; Nogawa, S.; Ito, D.; Suzuki, S.; Dembo, T.; Kosakai, A.; Fukuuchi, Y. Phosphorylation of cyclic adenosine monophosphate response element binding protein in oligodendrocytes in the corpus callosum after focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 2001, 21, 1177–1188. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Grammas, P. Insulin Resistance and Oligodendrocyte/Microvascular Endothelial Cell Dysfunction as Mediators of White Matter Degeneration in Alzheimer’s Disease. In Alzheimer’s Disease [Internet]; Wisniewski, T., Ed.; Codon Publications: Brisbane, Australia, 2019; Chapter 8; ISBN 978-0-6468096-8-7. [Google Scholar]
- Guo, Z.; Chen, Y.; Mao, Y.F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef]
- Prabhu, D.; Khan, S.M.; Blackburn, K.; Marshall, J.P.; Ashpole, N.M. Loss of insulin-like growth factor-1 signaling in astrocytes disrupts glutamate handling. J. Neurochem. 2019, 151, 689–702. [Google Scholar] [CrossRef]
- Logan, S.; Pharaoh, G.A.; Marlin, M.C.; Masser, D.R.; Matsuzaki, S.; Wronowski, B.; Yeganeh, A.; Parks, E.E.; Premkumar, P.; Farley, J.A.; et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol. Metab. 2018, 9, 141–155. [Google Scholar] [CrossRef]
- Garcia-Caceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.X.; et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, I.; García-Cáceres, C. Hypothalamic Astrocytes as a Specialized and Responsive Cell Population in Obesity. Int. J. Mol. Sci. 2021, 22, 6176. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Hernandez-Garzon, E.; Perez-Domper, P.; Perez-Alvarez, A.; Mederos, S.; Matsui, T.; Santi, A.; Trueba-Saiz, A.; Garcia-Guerra, L.; Pose-Utrilla, J.; et al. Insulin Regulates Astrocytic Glucose Handling Through Cooperation with IGF-I. Diabetes 2017, 66, 64–74. [Google Scholar] [CrossRef]
- Hernandez-Garzon, E.; Fernandez, A.M.; Perez-Alvarez, A.; Genis, L.; Bascunana, P.; Fernandez de la Rosa, R.; Delgado, M.; Angel Pozo, M.; Moreno, E.; McCormick, P.J.; et al. The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia 2016, 64, 1962–1971. [Google Scholar] [CrossRef]
- Albers, G.W.; Goldstein, L.B.; Hess, D.C.; Wechsler, L.R.; Furie, K.L.; Gorelick, P.B.; Hurn, P.; Liebeskind, D.S.; Nogueira, R.G.; Saver, J.L.; et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011, 42, 2645–2650. [Google Scholar] [CrossRef]
- Shapira, S.; Sapir, M.; Wengier, A.; Grauer, E.; Kadar, T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002, 925, 148–158. [Google Scholar] [CrossRef]
- He, Z.; Meschia, J.F.; Brott, T.G.; Dickson, D.W.; McKinney, M. Aging is neuroprotective during global ischemia but leads to increased caspase-3 and apoptotic activity in hippocampal neurons. Curr. Neurovasc. Res. 2006, 3, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Manwani, B.; Liu, F.; Xu, Y.; Persky, R.; Li, J.; McCullough, L.D. Functional recovery in aging mice after experimental stroke. Brain Behav. Immun. 2011, 25, 1689–1700. [Google Scholar] [CrossRef]
- Liu, F.; Benashski, S.E.; Persky, R.; Xu, Y.; Li, J.; McCullough, L.D. Age-related changes in AMP-activated protein kinase after stroke. Age 2012, 34, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y. Neuroprotective strategies for acute ischemic stroke: Recent progress and future perspectives. Precis. Future Med. 2017, 1, 115–121. [Google Scholar] [CrossRef]
- Tang, T.; Hu, L.; Liu, Y.; Fu, X.; Li, J.; Yan, F.; Cao, S.; Chen, G. Sex-Associated Differences in Neurovascular Dysfunction During Ischemic Stroke. Front. Mol. Neurosci. 2022, 15, 860959. [Google Scholar] [CrossRef]
- Alkayed, N.J.; Harukuni, I.; Kimes, A.S.; London, E.D.; Traystman, R.J.; Hurn, P.D. Gender-linked brain injury in experimental stroke. Stroke 1998, 29, 159–165. [Google Scholar] [CrossRef]
- Toung, T.J.; Traystman, R.J.; Hurn, P.D. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 1998, 29, 1666–1670. [Google Scholar] [CrossRef]
- Rassovsky, Y.; Levi, Y.; Agranov, E.; Sela-Kaufman, M.; Sverdlik, A.; Vakil, E. Predicting long-term outcome following traumatic brain injury (TBI). J. Clin. Exp. Neuropsychol. 2015, 37, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Røe, C.; Sveen, U.; Alvsåker, K.; Bautz-Holter, E. Post-concussion symptoms after mild traumatic brain injury: Influence of demographic factors and injury severity in a 1-year cohort study. Disabil. Rehabil. 2009, 31, 1235–1243. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Kling, A.; Henry, G.; Herndon, C.; Lavretsky, H. Local cerebral glucose metabolism in patients with long-term behavioral and cognitive deficits following mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 1996, 8, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Toth, A. Magnetic Resonance Imaging Application in the Area of Mild and Acute Traumatic Brain Injury: Implications for Diagnostic Markers? In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; Chapter 24; ISBN 978-1-4665-6598-2. [Google Scholar]
- Llompart-Pou, J.A.; Raurich, J.M.; Pérez-Bárcena, J.; Barceló, A.; Ibáñez, J.; Ayestarán, J.I. Acute Hypothalamic-pituitary-adrenal response in traumatic brain injury with and without extracerebral trauma. Neurocrit. Care 2008, 9, 230–236. [Google Scholar] [CrossRef]
- Shaughness, M.; Acs, D.; Brabazon, F.; Hockenbury, N.; Byrnes, K.R. Role of Insulin in Neurotrauma and Neurodegeneration: A Review. Front. Neurosci. 2020, 14, 547175. [Google Scholar] [CrossRef]
- Arora, P.; Singh, K.; Kumari, M.; Trivedi, R. Temporal profile of serum metabolites and inflammation following closed head injury in rats is associated with HPA axis hyperactivity. Metabolomics 2022, 18, 28. [Google Scholar] [CrossRef]
- Karelina, K.; Sarac, B.; Freeman, L.M.; Gaier, K.R.; Weil, Z.M. Traumatic brain injury and obesity induce persistent central insulin resistance. Eur. J. Neurosci. 2016, 43, 1034–1043. [Google Scholar] [CrossRef]
- Franklin, W.; Krishnan, B.; Taglialatela, G. Chronic synaptic insulin resistance after traumatic brain injury abolishes insulin protection from amyloid beta and tau oligomer-induced synaptic dysfunction. Sci. Rep. 2019, 9, 8228. [Google Scholar] [CrossRef]
- Selwyn, R.; Hockenbury, N.; Jaiswal, S.; Mathur, S.; Armstrong, R.C.; Byrnes, K.R. Mild traumatic brain injury results in depressed cerebral glucose uptake: An (18)FDG PET study. J. Neurotrauma 2013, 30, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119. [Google Scholar] [CrossRef]
- Brabazon, F.; Wilson, C.M.; Shukla, D.K.; Mathur, S.; Jaiswal, S.; Bermudez, S.; Byrnes, K.R.; Selwyn, R. [18F]FDG-PET Combined with MRI Elucidates the Pathophysiology of Traumatic Brain Injury in Rats. J. Neurotrauma 2017, 34, 1074–1085. [Google Scholar] [CrossRef]
- Yasmin, A.; Jokivarsi, K.; Poutiainen, P.; Pitkänen, A.; Gröhn, O.; Immonen, R. Chronic hypometabolism in striatum and hippocampal network after traumatic brain injury and their relation with memory impairment—[18F]-FDG-PET and MRI 4 months after fluid percussion injury in rat. Brain Res. 2022, 1788, 147934. [Google Scholar] [CrossRef] [PubMed]
- Brabazon, F.; Wilson, C.M.; Jaiswal, S.; Reed, J.; Frey, W.H., 2nd; Byrnes, K.R. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 3203–3218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Zou, J.; Cao, F. A meta-analysis of cohort studies: Traumatic brain injury and risk of Alzheimer’s Disease. PLoS ONE 2021, 16, e0253206. [Google Scholar] [CrossRef]
- Colca, J.R.; Finck, B.N. Metabolic Mechanisms Connecting Alzheimer’s and Parkinson’s Diseases: Potential Avenues for Novel Therapeutic Approaches. Front. Mol. Biosci. 2022, 9, 929328. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Pathophysiology of cerebral ischemia and brain trauma: Similarities and differences. J. Cereb. Blood Flow Metab. 2004, 24, 133–150. [Google Scholar] [CrossRef]
- Van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Prim. 2018, 4, 18003. [Google Scholar] [CrossRef]
- Nijssen, K.M.R.; Mensink, R.P.; Joris, P.J. Effects of Intranasal Insulin Administration on Cerebral Blood Flow and Cognitive Performance in Adults: A Systematic Review of Randomized, Placebo-Controlled Intervention Studies. Neuroendocrinology 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Blázquez, E.; Hurtado-Carneiro, V.; LeBaut-Ayuso, Y.; Velázquez, E.; García-García, L.; Gómez-Oliver, F.; Ruiz-Albusac, J.M.; Ávila, J.; Pozo, M.Á. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front. Endocrinol. 2022, 13, 873301. [Google Scholar] [CrossRef]
- Lee, T.H.; Yau, S.Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef]
- Sharma, S. High fat diet and its effects on cognitive health: Alterations of neuronal and vascular components of brain. Physiol. Behav. 2021, 240, 113528. [Google Scholar] [CrossRef] [PubMed]
- Al Haj Ahmad, R.M.; Ababneh, N.A.; Al-Domi, H.A. Brain insulin resistance as a mechanistic mediator links peripheral metabolic disorders with declining cognition. Diabetes Metab. Syndr. 2022, 16, 102468. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.J.; Singh, S.; Seksaria, S.; Das Gupta, G.; Singh, A. Inside the diabetic brain: Insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacol. Res. 2022, 182, 106358. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, K.; Chen, B.; Liu, R.; Cheng, S.; Zhang, Y.; Lu, J. Overnutrition Induced Cognitive Impairment: Insulin Resistance, Gut-Brain Axis, and Neuroinflammation. Front. Neurosci. 2022, 16, 884579. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Schulingkamp, R.J.; Pagano, T.C.; Hung, D.; Raffa, R.B. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci. Biobehav. Rev. 2000, 24, 855–872. [Google Scholar] [CrossRef]
- Heni, M.; Kullmann, S.; Preissl, H.; Fritsche, A.; Häring, H.U. Impaired insulin action in the human brain: Causes and metabolic consequences. Nat. Rev. Endocrinol. 2015, 11, 701–711. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Häring, H.U.; Fritsche, A.; Preissl, H. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.; Kessels, R.P.; de Haan, E.H.; Kappelle, L.J.; Biessels, G.J. Cerebral dysfunction in type 1 diabetes: Effects of insulin, vascular risk factors and blood-glucose levels. Eur. J. Pharmacol. 2004, 490, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Das, S.; Mahakkanukrauh, P. Blood-brain barrier dysfunction in ischemic stroke and diabetes: The underlying link, mechanisms and future possible therapeutic targets. Anat. Cell Biol. 2021, 54, 165–177. [Google Scholar] [CrossRef]
- Romanova, I.V.; Derkach, K.V.; Mikhrina, A.L.; Sukhov, I.B.; Mikhailova, E.V.; Shpakov, A.O. The Leptin, Dopamine and Serotonin Receptors in Hypothalamic POMC-Neurons of Normal and Obese Rodents. Neurochem. Res. 2018, 43, 821–837. [Google Scholar] [CrossRef]
- Derkach, K.; Zakharova, I.; Zorina, I.; Bakhtyukov, A.; Romanova, I.; Bayunova, L.; Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS ONE 2019, 14, e0213779. [Google Scholar] [CrossRef]
- Schuh, A.F.; Rieder, C.M.; Rizzi, L.; Chaves, M.; Roriz-Cruz, M. Mechanisms of brain aging regulation by insulin: Implications for neurodegeneration in late-onset Alzheimer’s disease. ISRN Neurol. 2011, 2011, 306905. [Google Scholar] [CrossRef] [PubMed]
- Matioli, M.N.P.S.; Nitrini, R. Mechanisms linking brain insulin resistance to Alzheimer’s disease. Dement. Neuropsychol. 2015, 9, 96–102. [Google Scholar] [CrossRef]
- Walker, J.M.; Harrison, F.E. Shared Neuropathological Characteristics of Obesity, Type 2 Diabetes and Alzheimer’s Disease: Impacts on Cognitive Decline. Nutrients 2015, 7, 7332–7357. [Google Scholar] [CrossRef]
- Athanasaki, A.; Melanis, K.; Tsantzali, I.; Stefanou, M.I.; Ntymenou, S.; Paraskevas, S.G.; Kalamatianos, T.; Boutati, E.; Lambadiari, V.; Voumvourakis, K.I.; et al. Type 2 Diabetes Mellitus as a Risk Factor for Alzheimer’s Disease: Review and Meta-Analysis. Biomedicines 2022, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.C.P.; Hallschmid, M. Outcomes and clinical implications of intranasal insulin administration to the central nervous system. Exp. Neurol. 2019, 317, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Korczyn, A.D.; Ali, S.S.; Bajenaru, O.; Choi, M.S.; Chopp, M.; Dermanovic-Dobrota, V.; Grünblatt, E.; Jellinger, K.A.; Kamal, M.A.; et al. The diabetic brain and cognition. J. Neural Transm. 2017, 124, 1431–1454. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cao, H.X.; Ke, D. Type 2 Diabetes Mellitus Easily Develops into Alzheimer’s Disease via Hyperglycemia and Insulin Resistance. Curr. Med. Sci. 2021, 41, 1165–1171. [Google Scholar] [CrossRef]
- Novak, V.; Milberg, W.; Hao, Y.; Munshi, M.; Novak, P.; Galica, A.; Manor, B.; Roberson, P.; Craft, S.; Abduljalil, A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 2014, 37, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, Y.; Manor, B.; Novak, P.; Milberg, W.; Zhang, J.; Fang, J.; Novak, V. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes 2015, 64, 1025–1034. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; He, G.; Gang, X.; Zhao, X.; Wang, G.; Ning, G. Cerebral perfusion alterations in type 2 diabetes mellitus—A systematic review. Front. Neuroendocrinol. 2021, 62, 100916. [Google Scholar] [CrossRef]
- Haan, M.N. Therapy Insight: Type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat. Clin. Pract. Neurol. 2006, 2, 159–166. [Google Scholar] [CrossRef]
- Chung, C.C.; Pimentel Maldonado, D.A.; Jor’dan, A.J.; Alfaro, F.J.; Lioutas, V.A.; Núñez, M.Z.; Novak, V. Lower cerebral vasoreactivity as a predictor of gait speed decline in type 2 diabetes mellitus. J. Neurol. 2018, 265, 2267–2276. [Google Scholar] [CrossRef]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Galindo-Mendez, B.; Trevino, J.A.; McGlinchey, R.; Fortier, C.; Lioutas, V.; Novak, P.; Mantzoros, C.S.; Ngo, L.; Novak, V. Memory advancement by intranasal insulin in type 2 diabetes (MemAID) randomized controlled clinical trial: Design, methods and rationale. Contemp. Clin. Trials 2020, 89, 105934. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.M.; Ferreira de Sá, D.S.; Westerhausen, R.; Strelzyk, F.; Larra, M.F.; Hallschmid, M.; Savaskan, E.; Oitzl, M.S.; Busch, H.P.; Naumann, E.; et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum. Brain Mapp. 2014, 35, 1944–1956. [Google Scholar] [CrossRef]
- Kullmann, S.; Veit, R.; Peter, A.; Pohmann, R.; Scheffler, K.; Häring, H.U.; Fritsche, A.; Preissl, H.; Heni, M. Dose-Dependent Effects of Intranasal Insulin on Resting-State Brain Activity. J. Clin. Endocrinol. Metab. 2018, 103, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Frank, S.; Heni, M.; Ketterer, C.; Veit, R.; Häring, H.U.; Fritsche, A.; Preissl, H. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology 2013, 97, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Veit, R.; Fritsche, L.; Häring, H.U.; Fritsche, A.; Birkenfeld, A.L.; Heni, M.; Preissl, H.; Kullmann, S. Sex differences in central insulin action: Effect of intranasal insulin on neural food cue reactivity in adults with normal weight and overweight. Int. J. Obes. 2022, 46, 1662–1670. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Gao, J.; Huang, Y.; Qi, F.; Lei, Y.; Ai, K.; Yan, X.; Cheng, M.; Su, Y.; et al. Altered Functional Connectivity of Insular Subregions in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 676624. [Google Scholar] [CrossRef]
- Heni, M.; Wagner, R.; Kullmann, S.; Veit, R.; Mat Husin, H.; Linder, K.; Benkendorff, C.; Peter, A.; Stefan, N.; Häring, H.U.; et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 2014, 63, 4083–4088. [Google Scholar] [CrossRef]
- Kullmann, S.; Fritsche, A.; Wagner, R.; Schwab, S.; Häring, H.U.; Preissl, H.; Heni, M. Hypothalamic insulin responsiveness is associated with pancreatic insulin secretion in humans. Physiol. Behav. 2017, 176, 134–138. [Google Scholar] [CrossRef]
- Wingrove, J.; Swedrowska, M.; Scherließ, R.; Parry, M.; Ramjeeawon, M.; Taylor, D.; Gauthier, G.; Brown, L.; Amiel, S.; Zelaya, F.; et al. Characterisation of nasal devices for delivery of insulin to the brain and evaluation in humans using functional magnetic resonance imaging. J. Control. Release 2019, 302, 140–147. [Google Scholar] [CrossRef]
- Ott, V.; Lehnert, H.; Staub, J.; Wönne, K.; Born, J.; Hallschmid, M. Central nervous insulin administration does not potentiate the acute glucoregulatory impact of concurrent mild hyperinsulinemia. Diabetes 2015, 64, 760–765. [Google Scholar] [CrossRef]
- Chistyakova, O.V.; Bondareva, V.M.; Shipilov, V.N.; Sukhov, I.B.; Shpakov, A.O. Intranasal administration of insulin eliminates the deficit of long-term spatial memory in rats with neonatal diabetes mellitus. Dokl. Biochem. Biophys. 2011, 440, 216–218. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Chistyakova, O.V.; Derkach, K.V.; Moiseyuk, I.V.; Bondareva, V.M. Intranasal insulin affects adenylyl cyclase system in rat tissues in neonatal diabetes. Cent. Eur. J. Biol. 2012, 7, 33–47. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Derkach, K.V.; Chistyakova, O.V.; Moiseyuk, I.V.; Sukhov, I.B.; Bondareva, V.M. Effect of intranasal insulin and serotonin on functional activity of the adenylyl cyclase system in myocardium, ovary, and uterus of rats with prolonged neonatal model of diabetes mellitus. J. Evol. Biochem. Physiol. 2013, 49, 153–164. [Google Scholar] [CrossRef]
- Shpakov, A.O.; Derkach, K.V.; Berstein, L.M. Brain signaling systems in the Type 2 diabetes and metabolic syndrome: Promising target to treat and prevent these diseases. Future Sci. OA 2015, 1, FSO25. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, I.B.; Shipilov, V.N.; Chistyakova, O.V.; Trost, A.M.; Shpakov, A.O. Long-term intranasal insulin administration improves spatial memory in male rats with prolonged type 1 diabetes mellitus and in healthy rats. Dokl. Biol. Sci. 2013, 453, 349–352. [Google Scholar] [CrossRef]
- Derkach, K.V.; Zorina, I.I.; Zakharova, I.O.; Basova, N.E.; Bakhtyukov, A.A.; Shpakov, A.O. The Influence of Intranasally Administered Insulin and C-peptide on AMP-Activated Protein Kinase Activity, Mitochondrial Dynamics and Apoptosis Markers in the Hypothalamus of Rats with Streptozotocin-Induced Diabetes. J. Evol. Biochem. Physiol. 2020, 56, 207–217. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bakhtyukov, A.A.; Basova, N.E.; Zorina, I.I.; Shpakov, A.O. The Restorative Effect of Combined Insulin and C-Peptide Intranasal Administration on Hormonal Status and Hypothalamic Signaling in the Male Rat Model of Severe Short-term Streptozotocin-Induced Diabetes. J. Evol. Biochem. Physiol. 2022, 58, 677–691. [Google Scholar] [CrossRef]
- Yosten, G.L.; Maric-Bilkan, C.; Luppi, P.; Wahren, J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E955–E968. [Google Scholar] [CrossRef]
- Yosten, G.L.; Kolar, G.R. The Physiology of Proinsulin C-Peptide: Unanswered Questions and a Proposed Model. Physiology 2015, 30, 327–332. [Google Scholar] [CrossRef]
- Souto, S.B.; Campos, J.R.; Fangueiro, J.F.; Silva, A.M.; Cicero, N.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Multiple Cell Signalling Pathways of Human Proinsulin C-Peptide in Vasculopathy Protection. Int. J. Mol. Sci. 2020, 21, 645. [Google Scholar] [CrossRef]
- Jörnvall, H.; Lindahl, E.; Astorga-Wells, J.; Lind, J.; Holmlund, A.; Melles, E.; Alvelius, G.; Nerelius, C.; Mäler, L.; Johansson, J. Oligomerization and insulin interactions of proinsulin C-peptide: Threefold relationships to properties of insulin. Biochem. Biophys. Res. Commun. 2010, 391, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Landreh, M.; Stukenborg, J.B.; Willander, H.; Söder, O.; Johansson, J.; Jörnvall, H. Proinsulin C-peptide interferes with insulin fibril formation. Biochem. Biophys. Res. Commun. 2012, 418, 489–493. [Google Scholar] [CrossRef]
- Shpakov, A.O. Mechanisms of action and therapeutic potential of proinsulin C-peptide. J. Evol. Biochem. Physiol. 2017, 53, 180–190. [Google Scholar] [CrossRef]
- Kolar, G.R.; Grote, S.M.; Yosten, G.L. Targeting orphan G protein-coupled receptors for the treatment of diabetes and its complications: C-peptide and GPR146. J. Intern. Med. 2017, 281, 25–40. [Google Scholar] [CrossRef]
- Lindfors, L.; Sundström, L.; Fröderberg Roth, L.; Meuller, J.; Andersson, S.; Kihlberg, J. Is GPR146 really the receptor for proinsulin C-peptide? Bioorg. Med. Chem. Lett. 2020, 30, 127208. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Perminova, A.A.; Buzanakov, D.M.; Shpakov, A.O. Intranasal administration of proinsulin C-peptide enhances the stimulating effect of insulin on insulin system activity in the hypothalamus of diabetic rats. Bull. Exp. Biol. Med. 2019, 167, 351–355. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Manjunatha, S.; Summer, P.; Gopala, S.; Zabeilski, P.; Dasari, S.; Vanderboom, P.M.; Lanza, I.R.; Klaus, K.A.; Nair, K.S. Insulin deficiency and intranasal insulin alter brain mitochondrial function: A potential factor for dementia in diabetes. FASEB J. 2019, 33, 4458–4472. [Google Scholar] [CrossRef]
- Torabi, N.; Noursadeghi, E.; Shayanfar, F.; Nazari, M.; Fahanik-Babaei, J.; Saghiri, R.; Khodagholi, F.; Eliassi, A. Intranasal insulin improves the structure-function of the brain mitochondrial ATP-sensitive Ca2+ activated potassium channel and respiratory chain activities under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166075. [Google Scholar] [CrossRef]
- Afolabi, S.O.; Folahan, J.; Agede, O.; Olorundare, O. Combined Intranasal Insulin/Saxagliptin/Metformin Therapies Ameliorate the Effect of Combined Oral Contraceptive- (COC-) Induced Metabolic Syndrome (MetS) with a Major Target on Glucose Metabolism in Adult Female Wistar Rats. Int. J. Reprod. Med. 2021, 2021, 9693171. [Google Scholar] [CrossRef]
- Caplan, G.A.; Lan, Z.; Newton, L.; Kvelde, T.; McVeigh, C.; Hill, M.A. Transcranial Doppler to measure cerebral blood flow in delirium superimposed on dementia. A cohort study. J. Am. Med. Dir. Assoc. 2014, 15, 355–360. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef] [PubMed]
- Caplan, G.A.; Kvelde, T.; Lai, C.; Yap, S.L.; Lin, C.; Hill, M.A. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer’s dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, L.R.; Nelson, J.A.; Wegner, E.A.; Caplan, G.A. 2-18F-fluoro-2-deoxyglucose positron emission tomography in delirium. J. Cereb. Blood Flow Metab. 2017, 37, 3556–3567. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liao, Y.; Mo, Y.; Li, Z.-R.; Liao, Q.; Wang, Y.-C.; Duan, K.-M.; Chen, M.-H.; Ouyang, W. Decreased cerebral glucose metabolism in elderly patients with postoperative delirium: A case-control study. J. Anesth. Perioper. Med. 2017, 4, 162–168. [Google Scholar] [CrossRef]
- Evered, L.; Silbert, B.; Knopman, D.S.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.S.; Crosby, G.; Berger, M.; Eckenhoff, R.G.; et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 2018, 121, 1005–1012. [Google Scholar] [CrossRef]
- Duning, T.; Ilting-Reuke, K.; Beckhuis, M.; Oswald, D. Postoperative delirium—Treatment and prevention. Curr. Opin. Anaesthesiol. 2021, 34, 27–32. [Google Scholar] [CrossRef]
- Fu, D.; Tan, X.; Zhang, M.; Chen, L.; Yang, J. Association between frailty and postoperative delirium: A meta-analysis of cohort study. Aging Clin. Exp. Res. 2022, 34, 25–37. [Google Scholar] [CrossRef]
- Needham, M.J.; Webb, C.E.; Bryden, D.C. Postoperative cognitive dysfunction and dementia: What we need to know and do. Br. J. Anaesth. 2017, 119, i115–i125. [Google Scholar] [CrossRef]
- Shoair, O.A.; Grasso Ii, M.P.; Lahaye, L.A.; Daniel, R.; Biddle, C.J.; Slattum, P.W. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: A prospective study. J. Anaesthesiol. Clin. Pharmacol. 2015, 31, 30–36. [Google Scholar] [CrossRef]
- Borozdina, A.; Qeva, E.; Cinicola, M.; Bilotta, F. Perioperative cognitive evaluation. Curr. Opin. Anaesthesiol. 2018, 31, 756–761. [Google Scholar] [CrossRef]
- Hermanides, J.; Qeva, E.; Preckel, B.; Bilotta, F. Perioperative hyperglycemia and neurocognitive outcome after surgery: A systematic review. Minerva Anestesiol. 2018, 84, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Badenes, R.; Qeva, E.; Giordano, G.; Romero-García, N.; Bilotta, F. Intranasal Insulin Administration to Prevent Delayed Neurocognitive Recovery and Postoperative Neurocognitive Disorder: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 2681. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Run, X.; Liang, Z.; Zhao, Y.; Dai, C.L.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front. Aging Neurosci. 2014, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, C.L.; Chen, Y.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal Insulin Prevents Anesthesia-Induced Spatial Learning and Memory Deficit in Mice. Sci. Rep. 2016, 6, 21186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Run, X.; Wei, Z.; Zeng, K.; Liang, Z.; Huang, F.; Ke, D.; Wang, Q.; Wang, J.Z.; Liu, R.; et al. Intranasal Insulin Prevents Anesthesia-induced Cognitive Impairments in Aged Mice. Curr. Alzheimer Res. 2019, 16, 8–18. [Google Scholar] [CrossRef]
- Yu, Q.; Dai, C.L.; Zhang, Y.; Chen, Y.; Wu, Z.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal Insulin Increases Synaptic Protein Expression and Prevents Anesthesia-Induced Cognitive Deficits Through mTOR-eEF2 Pathway. J. Alzheimers Dis. 2019, 70, 925–936. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, C.L.; Wu, Z.; Iqbal, K.; Liu, F.; Zhang, B.; Gong, C.X. Intranasal Insulin Prevents Anesthesia-Induced Cognitive Impairment and Chronic Neurobehavioral Changes. Front. Aging Neurosci. 2017, 9, 136. [Google Scholar] [CrossRef]
- Li, H.; Dai, C.L.; Gu, J.H.; Peng, S.; Li, J.; Yu, Q.; Iqbal, K.; Liu, F.; Gong, C.X. Intranasal Administration of Insulin Reduces Chronic Behavioral Abnormality and Neuronal Apoptosis Induced by General Anesthesia in Neonatal Mice. Front. Neurosci. 2019, 13, 706. [Google Scholar] [CrossRef]
- Dai, C.L.; Li, H.; Hu, X.; Zhang, J.; Liu, F.; Iqbal, K.; Gong, C.X. Neonatal Exposure to Anesthesia Leads to Cognitive Deficits in Old Age: Prevention with Intranasal Administration of Insulin in Mice. Neurotox. Res. 2020, 38, 299–311. [Google Scholar] [CrossRef]
- Wilder, R.T.; Flick, R.P.; Sprung, J.; Katusic, S.K.; Barbaresi, W.J.; Mickelson, C.; Gleich, S.J.; Schroeder, D.R.; Weaver, A.L.; Warner, D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009, 110, 796–804. [Google Scholar] [CrossRef]
- Flick, R.P.; Katusic, S.K.; Colligan, R.C.; Wilder, R.T.; Voigt, R.G.; Olson, M.D.; Sprung, J.; Weaver, A.L.; Schroeder, D.R.; Warner, D.O. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011, 128, e1053–e1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.; Feng, Y.; Yu, S.; Xu, C.; Chen, J.; Wang, T.; Xiao, W. General anesthesia in children and long-term neurodevelopmental deficits: A systematic review. Front. Mol. Neurosci. 2022, 15, 972025. [Google Scholar] [CrossRef] [PubMed]
- Vlisides, P.; Xie, Z. Neurotoxicity of general anesthetics: An update. Curr. Pharm. Des. 2012, 18, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Biddle, C.; Ford, V. The Neurotoxicity of General Anesthetic Drugs: Emphasis on the Extremes of Age. Annu. Rev. Nurs. Res. 2017, 35, 201–219. [Google Scholar] [CrossRef]
- Boscolo, A.; Starr, J.A.; Sanchez, V.; Lunardi, N.; DiGruccio, M.R.; Ori, C.; Erisir, A.; Trimmer, P.; Bennett, J.; Jevtovic-Todorovic, V. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: The importance of free oxygen radicals and mitochondrial integrity. Neurobiol. Dis. 2012, 45, 1031–1041. [Google Scholar] [CrossRef]
- Boscolo, A.; Milanovic, D.; Starr, J.A.; Sanchez, V.; Oklopcic, A.; Moy, L.; Ori, C.C.; Erisir, A.; Jevtovic-Todorovic, V. Early exposure to general anesthesia disturbs mitochondrial fission and fusion in the developing rat brain. Anesthesiology 2013, 118, 1086–1097. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. The Organizing Principles of Eukaryotic Ribosome Recruitment. Annu. Rev. Biochem. 2019, 88, 307–335. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell. Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Roque, P.S.; Hooshmandi, M.; Neagu-Lund, L.; Yin, S.; Yousefpour, N.; Sato, H.; Sato, T.; Nakadate, Y.; Kawakami, A.; Tahmasebi, S.; et al. Intranasal insulin rescues repeated anesthesia-induced deficits in synaptic plasticity and memory and prevents apoptosis in neonatal mice via mTORC1. Sci. Rep. 2021, 11, 15490. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Qin, F.; Yuan, L.; Lu, Z.; Nie, H.; Gong, G. Repeated Preoperative Intranasal Administration of Insulin Decreases the Incidence of Postoperative Delirium in Elderly Patients Undergoing Laparoscopic Radical Gastrointestinal Surgery: A Randomized, Placebo-Controlled, Double-Blinded Clinical Study. Am. J. Geriatr. Psychiatry 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Nitchingham, A.; Milne, A.; Toson, B.; Tuch, B.; Agar, M.; Close, J.; Caplan, G. Intranasal insulin for treatment of delirium in older hospitalised patients: Study protocol for a randomised controlled trial. BMJ Open 2021, 11, e050765. [Google Scholar] [CrossRef] [PubMed]
- Neirijnck, Y.; Papaioannou, M.D.; Nef, S. The Insulin/IGF System in Mammalian Sexual Development and Reproduction. Int. J. Mol. Sci. 2019, 20, 4440. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, H.; Fattahi, A.; Hamidian, G.; Sadigh-Eteghad, S.; Ziaee, M.; Mahmoudi, J. A closer look at the role of insulin for the regulation of male reproductive function. Gen. Comp. Endocrinol. 2021, 300, 113643. [Google Scholar] [CrossRef]
- Griffeth, R.J.; Carretero, J.; Burks, D.J. Insulin receptor substrate 2 is required for testicular development. PLoS ONE 2013, 8, e62103. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.; Yan, L.; Yong, J.; Liu, X.; Hu, Y.; Fan, X.; Wu, X.; Guo, H.; Wang, X.; et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 2017, 20, 858–873.e4. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Sliwowska, J.H.; Fergani, C.; Gawałek, M.; Skowronska, B.; Fichna, P.; Lehman, M.N. Insulin: Its role in the central control of reproduction. Physiol. Behav. 2014, 133, 197–206. [Google Scholar] [CrossRef]
- Rønnekleiv, O.K.; Qiu, J.; Kelly, M.J. Arcuate Kisspeptin Neurons Coordinate Reproductive Activities with Metabolism. Semin. Reprod. Med. 2019, 37, 131–140. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Kaltsas, G.; Grossman, A.B.; Randeva, H.S.; Weickert, M.O. Mechanisms of Central Hypogonadism. Int. J. Mol. Sci. 2021, 22, 8217. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Dosouto, C.; Calaf, J.; Polo, A.; Haahr, T.; Humaidan, P. Growth Hormone and Reproduction: Lessons Learned From Animal Models and Clinical Trials. Front. Endocrinol. 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D. Aging and hormones of the hypothalamo-pituitary axis: Gonadotropic axis in men and somatotropic axes in men and women. Ageing Res. Rev. 2008, 7, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Carlomagno, F.; Cangiano, B.; Kanakis, G.; Pozza, C.; Sbardella, E.; Isidori, A.M.; Krausz, C.; Gianfrilli, D. Somatotropic-Testicular Axis: A crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology 2021, 9, 168–184. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Andlib, N.; Sajad, M.; Kumar, R.; Thakur, S.C. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: A mechanistic insight. Acta Histochem. 2022, 125, 151974. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P. Insulin Resistance and Polycystic Ovary Syndrome. Curr. Pharm. Des. 2016, 22, 5526–5534. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, M.; Kharwar, R.K.; Roy, V.K. Synthetic leptin c-fragment peptide minimises heat-induced impairment of spermatogenesis in mice via Stat3 signalling. Theriogenology 2022, 178, 40–49. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bondareva, V.M.; Shpakov, A.O. Vliianie intranazal’no vvodimogo insulina na metabolicheskie i gormonal’nye pokazateli u vzroslykh samtsov krys, narushennye vsledstvie trekhdnevnogo golodaniia v rannem postnatal’nom periode [Influence of intranasally administered insulin on metabolic and hormonal parameters in adult male rats, impaired due to three-day fasting in the early postnatal period]. Biomed. Khim. 2022, 68, 263–271. (In Russian) [Google Scholar] [CrossRef]
- McCarty, M.F. Central insulin may up-regulate thyroid activity by suppressing neuropeptide Y release in the paraventricular nucleus. Med. Hypotheses 1995, 45, 193–199. [Google Scholar] [CrossRef]
- Fekete, C.; Singru, P.S.; Sanchez, E.; Sarkar, S.; Christoffolete, M.A.; Riberio, R.S.; Rand, W.M.; Emerson, C.H.; Bianco, A.C.; Lechan, R.M. Differential effects of central leptin, insulin, or glucose administration during fasting on the hypothalamic-pituitary-thyroid axis and feeding-related neurons in the arcuate nucleus. Endocrinology 2006, 147, 520–529. [Google Scholar] [CrossRef]
- Fekete, C.; Kelly, J.; Mihály, E.; Sarkar, S.; Rand, W.M.; Légrádi, G.; Emerson, C.H.; Lechan, R.M. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 2001, 142, 2606–2613. [Google Scholar] [CrossRef]
- Fekete, C.; Sarkar, S.; Rand, W.M.; Harney, J.W.; Emerson, C.H.; Bianco, A.C.; Lechan, R.M. Agouti-related protein (AGRP) has a central inhibitory action on the hypothalamic-pituitary-thyroid (HPT) axis; comparisons between the effect of AGRP and neuropeptide Y on energy homeostasis and the HPT axis. Endocrinology 2002, 143, 3846–3853. [Google Scholar] [CrossRef]
- Nillni, E.A. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front. Neuroendocrinol. 2010, 31, 134–156. [Google Scholar] [CrossRef]
- Kouidhi, S.; Clerget-Froidevaux, M.S. Integrating Thyroid Hormone Signaling in Hypothalamic Control of Metabolism: Crosstalk Between Nuclear Receptors. Int. J. Mol. Sci. 2018, 19, 2017. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Han, Y.; Teng, W.; Shan, Z. Correlation Between Thyroid Nodules and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 730279. [Google Scholar] [CrossRef]
- Frommer, L.; Kahaly, G.J. Type 1 Diabetes and Autoimmune Thyroid Disease-The Genetic Link. Front. Endocrinol. 2021, 12, 618213. [Google Scholar] [CrossRef]
- Boelen, A.; van Trotsenburg, A.S.P.; Fliers, E. Congenital isolated central hypothyroidism: Novel mutations and their functional implications. Handb. Clin. Neurol. 2021, 180, 161–169. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shpakov, A.O.; Zorina, I.I.; Derkach, K.V. Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. Int. J. Mol. Sci. 2023, 24, 3278. https://doi.org/10.3390/ijms24043278
Shpakov AO, Zorina II, Derkach KV. Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. International Journal of Molecular Sciences. 2023; 24(4):3278. https://doi.org/10.3390/ijms24043278
Chicago/Turabian StyleShpakov, Alexander O., Inna I. Zorina, and Kira V. Derkach. 2023. "Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium" International Journal of Molecular Sciences 24, no. 4: 3278. https://doi.org/10.3390/ijms24043278
APA StyleShpakov, A. O., Zorina, I. I., & Derkach, K. V. (2023). Hot Spots for the Use of Intranasal Insulin: Cerebral Ischemia, Brain Injury, Diabetes Mellitus, Endocrine Disorders and Postoperative Delirium. International Journal of Molecular Sciences, 24(4), 3278. https://doi.org/10.3390/ijms24043278