Enzyme Conditioning of Chicken Collagen and Taguchi Design of Experiments Enhancing the Yield and Quality of Prepared Gelatins
Abstract
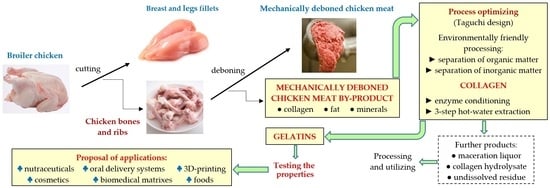
:1. Introduction
2. Results
2.1. Mass Balance of the Process
2.2. First Gelatin Fractions
2.3. Second Gelatin Fractions
2.4. Third Gelatin Fractions
3. Discussion
3.1. Comparing and Contrasting Results with References
3.1.1. Technological Conditions for Gelatin Preparation and Gelatin Yield
3.1.2. Gel Strength, Meting Point and Gelling Point
3.1.3. Viscosity, Ash, Water Holding Capacity and Fat Binding Capacity
3.1.4. Foaming Capacity, Foaming Stability, Emulsifying Capacity, and Emulsion Stability
3.2. Discussion Summary
4. Materials and Methods
4.1. Materials, Appliances and Chemicals
4.2. Experimental Design and Statistical Analysis
4.3. Processing of MDCM By-Product into Gelatins
4.4. Analytical Part
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries, review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 163–295. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Gelatin Market Analysis, Grand View Research, Inc.: San Francisco, CA, USA, 2019; Excel file.
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Choe, J.; Kim, H.Y. Effects of chicken feet gelatin extracted at different temperatures and wheat fiber with different particle sizes on the physicochemical properties of gels. Poult. Sci. 2018, 97, 1082–1088. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Nazlin, F.B.; Howell, K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Kim, T.K.; Ham, Y.K.; Shin, D.M.; Kim, H.W.; Jang, H.W.; Kim, Y.B.; Choi, Y.S. Extraction of crude gelatin from duck skin: Effects of heating methods on gelatin yield. Poult. Sci. 2020, 99, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Extraction and characterization of gelatin from the feet of Pekin duck (Anas platyrhynchos domestica) as affected by acid, alkaline, and enzyme pretreatment. Int. J. Biol. Macromol. 2017, 98, 586–594. [Google Scholar] [CrossRef]
- Saenmuang, S.; Phothiset, S.; Chumnanka, C. Extraction and characterization of gelatin from black-bone chicken by-products. Food Sci. Biotechnol. 2020, 29, 469–478. [Google Scholar] [CrossRef]
- Yuliani, D.; Awalsasi, D.; Jannah, A. Characterization of gelatin profile of chicken broiler (Gallus domestica) bone using SDS-PAGE electrophoresis. Alchemy–J. Chem. 2019, 7, 7–12. [Google Scholar] [CrossRef]
- Du, L.; Khiari, Z.; Pietrasik, Z.; Betti, M. Physicochemical and functional properties of gelatins extracted from turkey and chicken heads. Poult. Sci. 2013, 92, 2463–2474. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, R.; Ding, M.; Tao, L.; Liu, L.; Tao, N.; Wang, X.; Zhong, J. Effect of extraction methods on the structural characteristics, functional properties, and emulsion stabilization ability of tilapia skin gelatins. Food Chem. 2020, 328, 127114. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.V.; Shamasundar, B.A. Rheological properties of gelatin prepared from the swim bladders of freshwater fish Catla catla. Food Hydrocoll. 2015, 48, 47–54. [Google Scholar] [CrossRef]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT-Food Sci. Technol. 2014, 55, 579–585. [Google Scholar] [CrossRef]
- Liu, H.Y.; Han, J.; Guo, S.D. Characteristics of the gelatin extracted from Channel Catfish (Ictalurus Punctatus) head bones. LWT-Food Sci. Technol. 2009, 42, 540–544. [Google Scholar] [CrossRef]
- Jamilah, B.; Tan, K.W.; Umi Hartina, M.R.; Azizah, A. Gelatins from three cultured freshwater fish skins obtained by liming process. Food Hydrocoll. 2011, 25, 1256–1260. [Google Scholar] [CrossRef]
- Tümerkan, E.T.A.; Cansu, Ü.; Boran, G.; Regenstein, J.M.; Özoğul, F. Physiochemical and functional properties of gelatin obtained from tuna, frog and chicken skins. Food Chem. 2019, 287, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem. 2014, 152, 276–284. [Google Scholar] [CrossRef]
- Jridi, M.; Nasri, R.; Lassoued, I.; Souissi, N.; Mbarek, A.; Barkia, A.; Nasri, M. Chemical and biophysical properties of gelatins extracted from alkali-pretreated skin of cuttlefish (Sepia officinalis) using pepsin. Food Res. Int. 2013, 54, 1680–1687. [Google Scholar] [CrossRef]
- Badway, H.M.R.; Abd El-Moniem, S.M.; Soliman, A.M.; Rabie, M.A. Physicochemical properties of gelatin extracted from Nile tilapia (Oreochromis niloticus) and Nile perch (Lates niloticus) fish skins. Zagazig J. Agric. Res. 2019, 46, 1529–1537. [Google Scholar] [CrossRef]
- Peterková, P.; Lapčík, L., Jr. Collagen—Properties, modifications and applications. Chem. Listy 2000, 94, 371–379. (In Czech) [Google Scholar]
- Ma, Y.; Zeng, Y.; Ma, X.; Yang, R.; Zhao, W. A simple and eco-friendly method of gelatin production from bone: One-step biocatalysis. J. Clean. Prod. 2019, 209, 916–926. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.L.S.; Fontes, P.R. Effects of the addition of mechanically deboned poultry meat and collagen fibers on quality characteristics of frankfurter-type sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.; Mor-Mur, M.; Capellas, M.; Guamis, B.; Pla, R. Mechanically recovered poultry meat sausages manufactured with high hydrostatic pressure. Poult. Sci. 1999, 78, 914–921. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Mechanical Deboning. In Handbook of Meat and Meat Processing; Hui, Y.H., Aalhus, J.L., Cocolin, L., Guerrero-Legarreta, I., Nollet, L.M., Purchas, R.W., Schilling, M.W., Stanfield, P., Xiong, Y.L., Eds.; Taylor & Francis Inc: Abingdon, UK, 2012; Chapter 24. [Google Scholar]
- Daros, F.G.; Masson, M.L.; Amico, S.C. The influence of the addition of mechanically deboned poultry meat on the rheological properties of sausage. J. Food Eng. 2005, 68, 185–189. [Google Scholar] [CrossRef]
- Massingue, A.A.; de Almeida Torres, F.R.; Fontes, P.R.; de Lemos, S.R.A.; Fontes, E.A.F.; Perez, J.R.O.; Ramos, E.M. Effect of mechanically deboned poultry meat content on technological properties and sensory characteristics of lamb and mutton sausages. Asian-Australas J. Anim. Sci. 2018, 31, 576–584. [Google Scholar] [CrossRef]
- Cheow, C.S.; Norizah, M.S.; Kyaw, Z.Y.; Howell, N.K. Preparation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma). Food Chem. 2007, 101, 386–391. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Eysturskarð, J.; Haug, I.J.; Elharfaoui, N.; Djabourov, M.; Draget, K.I. Structural and mechanical properties of fish gelatin as a function of extraction conditions. Food Hydrocoll. 2009, 23, 1702–1711. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci. Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Ogawa, M.; Portier, R.J.; Moody, M.W.; Bell, J.; Schexnayder, M.A.; Losso, J.N. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus). Food Chem. 2004, 88, 495–501. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y. Effects of concentration on nanostructural images and physical properties of gelatin from channel catfish skins. Food Hydrocoll. 2009, 23, 577–584. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish—Black tilapia (Oreochromis mossambicus) and red tilapia (Orchromis nilotica). Food Chem. 2002, 77, 81–84. [Google Scholar] [CrossRef]
- Saxena, A.; Sachin, K.; Bohidar, H.B.; Verma, A.K. Effect of molecular weight heterogeneity on drug encapsulation efficiency of gelatin nano-particles. Colloids Surf. B Biointerfaces 2005, 45, 42–48. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatifrom skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Badii, F.; Howell, N.K. Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006, 20, 630–640. [Google Scholar] [CrossRef]
- Mokrejš, P.; Gál, R.; Pavlačková, J.; Janáčová, D. Valorization of a by-product from the production of mechanically deboned chicken meat for preparation of gelatins. Molecules 2021, 26, 349. [Google Scholar] [CrossRef]
- Rafieian, F.; Keramat, J.; Kadivar, M. Optimization of gelatin extraction from chicken deboner residue using RSM method. J. Food Sci. Technol. 2011, 50, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Rammaya, K.; Ying, V.Q.; Babji, A.S. Physicochemical analysis of gelatin extracted from mechanically deboned chicken meat (mdcm) residue. Int. J. Food Saf. Nutr. Publ. Health 2012, 5, 147–167. [Google Scholar] [CrossRef]
- Erge, A.; Zorba, Ö. Optimization of gelatin extraction from chicken mechanically deboned meat residue using alkaline pre-treatment. LWT-Food Sci. Technol. 2018, 97, 205–212. [Google Scholar] [CrossRef]
- Al-Kahtani, H.A.; Jaswir, I.; Ismail, E.A.; Ahmed, M.A.; Monsur Hammed, A.; Olorunnisola, S.; Octavianti, F. Structural characteristics of camel-bone gelatin by demineralization and extraction. Int. J. Food Prop. 2016, 20, 2559–2568. [Google Scholar] [CrossRef]
- Shyni, K.; Hema, G.S.; Ninan, G.; Mathew, S.; Joshy, C.G.; Lakshmanan, P.T. Isolation and characterization of gelatin from the skins of skipjack tuna (Katsuwonus pelamis), dog shark (Scoliodon sorrakowah), and rohu (Labeo rohita). Food Hydrocoll. 2014, 39, 68–76. [Google Scholar] [CrossRef]
- Roy, B.C.; Das, C.; Hong, H.; Betti, M.; Bruce, H.L. Extraction and characterization of gelatin from bovine heart. Food Biosci. 2017, 20, 116–124. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.0. European Directorate for the Quality of Medicines & Health Care, Strasbourgh, France. 2019. Available online: https://www.scribd.com/document/508063535/European-Pharmacopoeia-10-0# (accessed on 18 December 2022).
- Food Chemical Codex 12. Available online: https://www.foodchemicalscodex.org/ (accessed on 24 June 2022).
- Nollet, L.M.L.; Toldrá, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 357–754. [Google Scholar] [CrossRef]
- ISO 3496:1994; Meat and Meat Products—Determination of Hydroxyproline Content. ISO. Available online: https://cdn.standards.iteh.ai/samples/8848/908d030b1d6a4807bc2ac15fee8d51f9/SIST-ISO-3496-1995.pdf (accessed on 16 July 2022).
- Vázquez-Ortiz, F.A.; González-Méndez, N.F. Determination of collagen as a quality index in Bologna from Northwestern Mexico. J. Food Compos. Anal. 1996, 9, 269–276. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 2nd ed.; Elsevier: London, UK, 2014; pp. 33–85. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Chen, J.C.; Kirby, E.D. Surface roughness optimization in an end-milling operation using the Taguchi design method. J. Mater. Process. Technol. 2007, 184, 233–239. [Google Scholar] [CrossRef]
- Standard Testing Methods for Edible Gelatin. Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Available online: http://www.gelatin-gmia.com/images/GMIA_Official_Methods_of_Gelatin_Revised_2013.pdf (accessed on 21 June 2022).
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-chemical characterization of culled plantain pulp starch, peel starch and flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Li, F.; Jia, D.; Yao, K. Amino acid composition and functional properties of collagen polypeptide from yak (Bos grunniens) bone. LWT-Food Sci. Technol. 2009, 42, 945–949. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional properties of lupin seed (Supinus mutabilis) proteins and protein concentrates. J. Food Sci. 1982, 47, 491–497. [Google Scholar] [CrossRef]
- Neto, V.Q.; Narain, N.; Silva, J.B.; Bora, P.S. Functional properties of raw and heat processed cashew nut (Anarcardium occidentale L.) kernel protein isolates. Die Nahrung 2001, 45, 258–262. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yazdani-Dehnavi, M.; Mirzapour-Kouhdasht, A. The effects of enzymatically aided acid-swelling process on gelatin extracted from fish by- products. Food Sci. Nutr. 2020, 8, 5017–5025. [Google Scholar] [CrossRef] [PubMed]



| Exp. No. | Factor A (°C) | Factor B (min) | YH (%) | YG1 (%) | YG2 (%) | YG3 (%) | UR (%) | MBE (%) | YG∑ (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | 20 | 10.6 | 6.2 | 39.7 | 8.0 | 32.6 | 2.9 | 53.9 |
| 2 | 42 | 40 | 12.0 | 22.5 | 44.8 | 3.2 | 14.2 | 3.3 | 70.5 |
| 3 | 42 | 60 | 11.1 | 25.4 | 49.3 | 2.1 | 8.9 | 3.2 | 76.8 |
| 4 | 46 | 20 | 12.4 | 45.4 | 24.2 | 2.9 | 11.8 | 3.3 | 72.5 |
| 5 | 46 | 40 | 11.7 | 38.4 | 30.4 | 4.0 | 12.2 | 3.3 | 72.8 |
| 6 | 46 | 60 | 11.0 | 19.2 | 49.8 | 3.9 | 14.6 | 1.5 | 72.9 |
| 7 | 50 | 20 | 12.1 | 29.1 | 30.8 | 7.2 | 17.8 | 3.0 | 67.1 |
| 8 | 50 | 40 | 11.6 | 30.5 | 27.1 | 7.3 | 21.1 | 2.4 | 64.9 |
| 9 | 50 | 60 | 10.8 | 32.8 | 22.4 | 7.2 | 21.9 | 4.9 | 62.4 |
| 10 * | 46 | 40 | 3.3 | 1.3 | 2.4 | 4.4 | 86.9 | 1.7 | 8.1 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-Value | p-Value | |
|---|---|---|---|---|---|
| Response: The yield of the 1st gelatin fraction, YG1 (%) = −44.6 + 1.60A − 0.028B | |||||
| Regression | 2 | 246.30 | 123.148 | 0.94 | 0.441 |
| Factor A (Extraction temperature) | 1 | 244.48 | 244.482 | 1.87 | 0.220 |
| Factor B (Extraction time) | 1 | 1.82 | 1.815 | 0.01 | 0.910 |
| Error | 6 | 784.12 | 130.686 | ||
| Total | 8 | 1030.42 | |||
| Response: The yield of the 2nd gelatin fraction, YG2 (%) = 129.0 − 2.229A + 0.223B | |||||
| Regression | 2 | 596.7 | 298.37 | 5.59 | 0.043 |
| Factor A (Extraction temperature) | 1 | 477.0 | 477.04 | 8.94 | 0.024 ● |
| Factor B (Extraction time) | 1 | 119.7 | 119.71 | 2.24 | 0.185 |
| Error | 6 | 320.2 | 53.36 | ||
| Total | 8 | 916.9 | |||
| Response: The yield of the 3rd gelatin fraction, YG3 (%) = −9.4 + 0.350A − 0.0408B | |||||
| Regression | 2 | 15.762 | 7.881 | 1.79 | 0.246 |
| Factor A (Extraction temperature) | 1 | 11.760 | 11.760 | 2.67 | 0.153 |
| Factor B (Extraction time) | 1 | 4.002 | 4.002 | 0.91 | 0.377 |
| Error | 6 | 26.407 | 4.401 | ||
| Total | 8 | 42.169 | |||
| Process Factors | Gelatin Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | Factor A (°C) | Factor B (min) | Ash (%) | υ (mPa·s) | WHC (%) | FBC (%) | FC (%) | FS (%) | EC (%) | ES (%) |
| 1 | 42 | 20 | 1.17 | 1.7 | 220 | 840 | 8 | 0 | 47 | 93 |
| 2 | 42 | 40 | 0.97 | 1.6 | 220 | 900 | 8 | 2 | 47 | 92 |
| 3 | 42 | 60 | 1.23 | 1.5 | 230 | 920 | 7 | 2 | 48 | 93 |
| 4 | 46 | 20 | 0.88 | 1.6 | 230 | 1090 | 6 | 2 | 48 | 93 |
| 5 | 46 | 40 | 0.96 | 1.5 | 230 | 1090 | 7 | 2 | 48 | 94 |
| 6 | 46 | 60 | 1.43 | 1.5 | 240 | 1110 | 7 | 2 | 47 | 93 |
| 7 | 50 | 20 | 1.39 | 1.5 | 240 | 1140 | 8 | 3 | 47 | 93 |
| 8 | 50 | 40 | 1.02 | 1.4 | 250 | 1210 | 8 | 4 | 48 | 95 |
| 9 | 50 | 60 | 1.16 | 1.4 | 240 | 1210 | 7 | 3 | 46 | 94 |
| 10 * | 46 | 40 | 1.02 | 1.6 | 240 | 1150 | 7 | 4 | 48 | 93 |
| Process Factors | Gelatin Properties | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | Factor A (°C) | Factor B (min) | GS (Bloom) | MP (°C) | GP (°C) | υ (mPa·s) | Ash (%) | WHC (%) | FBC (%) | FC (%) | FS (%) | EC (%) | ES (%) |
| 1 | 42 | 20 | 174 | 35.3 | 16.6 | 2.2 | 0.66 | 1010 | 1310 | 20 | 16 | 48 | 93 |
| 2 | 42 | 40 | 80 | 28.9 | 15.0 | 1.6 | 0.35 | 930 | 980 | 18 | 8 | 46 | 93 |
| 3 | 42 | 60 | 125 | 32.3 | 15.3 | 1.8 | 0.34 | 970 | 1390 | 18 | 10 | 47 | 93 |
| 4 | 46 | 20 | 143 | 32.8 | 15.5 | 1.9 | 0.43 | 950 | 1070 | 22 | 18 | 48 | 93 |
| 5 | 46 | 40 | 105 | 30.4 | 14.9 | 2.0 | 0.36 | 960 | 1170 | 20 | 8 | 51 | 90 |
| 6 | 46 | 60 | 262 | 36.8 | 17.1 | 2.9 | 0.45 | 960 | 1230 | 20 | 16 | 48 | 93 |
| 7 | 50 | 20 | 284 | 37.9 | 16.7 | 2.7 | 0.46 | 980 | 1250 | 20 | 16 | 47 | 93 |
| 8 | 50 | 40 | 269 | 35.1 | 16.4 | 2.6 | 0.43 | 990 | 1470 | 20 | 18 | 49 | 90 |
| 9 | 50 | 60 | 290 | 38.4 | 17.6 | 3.8 | 0.70 | 1090 | 1460 | 36 | 24 | 52 | 92 |
| 10 * | 46 | 40 | 460 | 35.1 | 26.8 | 6.8 | 0.54 | 1320 | 1540 | 40 | 28 | 54 | 93 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-value | p-Value | |
|---|---|---|---|---|---|
| Response: Gel strength (Bloom) = −723 + 19.33A + 0.64B | |||||
| Regression | 2 | 36,870.8 | 18,435.4 | 5.69 | 0.041 |
| Factor A (Extraction temperature) | 1 | 35,882.7 | 35,882.7 | 11.07 | 0.016 ● |
| Factor B (Extraction time) | 1 | 988.2 | 988.2 | 0.30 | 0.601 |
| Error | 6 | 19,451.2 | 3241.9 | ||
| Total | 8 | 56,322.0 | |||
| Response: Viscosity (mPa·s) = −4.89 + 0.1458A + 0.01417B | |||||
| Regression | 2 | 2.5233 | 1.2617 | 5.98 | 0.037 |
| Factor A (Extraction temperature) | 1 | 2.0417 | 2.0417 | 9.68 | 0.021 ● |
| Factor B (Extraction time) | 1 | 0.4817 | 0.4817 | 2.28 | 0.182 |
| Error | 6 | 1.2656 | 0.2109 | ||
| Total | 8 | 3.7889 | |||
| Response: Meting point (°C) = 5.2 + 0.621A + 0.0125B | |||||
| Regression | 2 | 37.3767 | 18.6883 | 2.21 | 0.191 |
| Factor A (Extraction temperature) | 1 | 37.0017 | 37.0017 | 4.37 | 0.082 |
| Factor B (Extraction time) | 1 | 0.3750 | 0.3750 | 0.04 | 0.840 |
| Error | 6 | 50.8322 | 8.4720 | ||
| Total | 8 | 88.2089 | |||
| Response: Gelling point (°C) = 8.44 + 0.1583A + 0.0100B | |||||
| Regression | 2 | 2.6467 | 1.3233 | 1.60 | 0.277 |
| Factor A (Extraction temperature) | 1 | 2.4067 | 2.4067 | 2.92 | 0.138 |
| Factor B (Extraction time) | 1 | 0.2400 | 0.2400 | 0.29 | 0.609 |
| Error | 6 | 4.9489 | 0.8248 | ||
| Total | 8 | 7.5956 | |||
| Process Factors | Gelatin Properties | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | Factor A (°C) | Factor B (min) | GS (Bloom) | MP (°C) | GP (°C) | υ (mPa·s) | Ash (%) | WHC (%) | FBC (%) | FC (%) | FS (%) | EC (%) | ES (%) |
| 1 | 42 | 20 | 80 | 29.2 | 14.9 | 1.7 | 0.67 | 550 | 1190 | 7 | 0 | 48 | 95 |
| 2 | 42 | 40 | 82 | 30.1 | 15.0 | 1.7 | 0.35 | 560 | 1180 | 6 | 0 | 47 | 97 |
| 3 | 42 | 60 | 88 | 30.8 | 15.3 | 1.8 | 0.81 | 680 | 1220 | 8 | 0 | 48 | 97 |
| 4 | 46 | 20 | 0 | NA | NA | 1.4 | 0.96 | 220 | 1060 | 20 | 12 | 48 | 96 |
| 5 | 46 | 40 | 0 | NA | NA | 1.5 | 0.71 | 210 | 990 | 18 | 11 | 47 | 96 |
| 6 | 46 | 60 | 0 | NA | NA | 1.5 | 0.67 | 220 | 1040 | 20 | 12 | 48 | 95 |
| 7 | 50 | 20 | 223 | 33.9 | 16.0 | 2.4 | 0.59 | 680 | 1100 | 17 | 0 | 47 | 96 |
| 8 | 50 | 40 | 225 | 34.4 | 16.2 | 2.4 | 0.65 | 680 | 1120 | 16 | 0 | 48 | 96 |
| 9 | 50 | 60 | 230 | 34.8 | 16.5 | 2.6 | 0.48 | 730 | 1130 | 16 | 0 | 48 | 97 |
| 10* | 46 | 40 | 245 | 34.1 | 15.2 | 2.4 | 0.63 | 910 | 1220 | 19 | 0 | 47 | 91 |
| Conditions for Processing Collagen Raw Material into Gelatins | Gelatin Yield (%) |
|---|---|
| Current study. Collagen tissue: MDCM by-product | 53.9–76.8 |
| Separation of non-collagenous matter: 0.2 mol/L NaCl, 0.03 mol/L NaOH; defatting: petroleum ether + ethanol (22 °C, 48 h); demineralization: 3.0% HCl (22 °C, 96 h); washing with H2O (22 °C); conditioning: protease (pH 6.5–7.0, 24 h, 22 °C); filtration; washing with H2O; 1st gelatin extraction: H2O, 42–50 °C, 40–60 min; filtration; 2nd gelatin extraction: H2O, 65 °C, 30 min; filtration; 3rd gelatin extraction: 80 °C, 30 min; gelatin drying: 40 °C, 12 h and 65 °C, 8 h. | |
| [41]. Collagen tissue: MDCM by-product | 23.2–38.6 |
| Separation of non-collagenous matter: H2O, 0.2 mol/L NaCl, 0.03 mol/L NaOH; defatting: lipolytic enzyme (22 °C, 48 h), petroleum ether + ethanol (22 °C, 20 h); conditioning: protease (pH 6.5–7.0, 24–72 h, 22 °C); filtration; washing with H2O; 1st gelatin extraction: H2O, 64–80 °C, 60–180 min; filtration; 2nd gelatin extraction: H2O, 90 °C, 120 min; filtration; gelatin drying: 50 °C, 48 h. | |
| [42]. Collagen tissue: MDCM by-product | 1.6–16.9 |
| Defatting: hexane; washing with H2O (22 °C); separation of non-collagenous matter: 1% NaCl (pH 10.5–10.7, 22 °C, 30 min); filtration; conditioning: 1.64–8.36% HCl (22 °C, 24 h); filtration; washing with H2O; neutralisation to pH 6–7; gelatin extraction: 53–87 °C, 2–12 h; ion exchange: Purolite C-100-E; gelatin drying: 40–42 °C. | |
| [43]. Collagen tissue: MDCM by-product | 6.0–16.0 |
| Defatting: H2O (35 °C); washing with H2O (25 °C); demineralization: 3.0% HCl (10 °C, 24 h); washing with H2O (22 °C); conditioning: 4.0% NaOH (22 °C, 72 h); filtration; washing with H2O (25 °C); neutralisation: H3PO4 (22 °C); gelatin extraction: 60–80 °C, 2–12 h, pH 4.0; filtration; centrifugation (22 °C, 30 min); gelatin freeze drying. | |
| [44]. Collagen tissue: MDCM by-product | 6.0–15.0 |
| Separation of non-collagenous matter: H2O (35 °C, 1 h); washing with H2O; demineralization: 3.0% HCl (10 °C, 24 h); washing with H2O; conditioning: 2.0–4.2% NaOH (22 °C, 48 h); washing with H2O; neutralisation: H3PO4 (22 °C, pH 4.0); washing with H2O; gelatin extraction: 60–82 °C, 50–250 min; filtration; centrifugation (22 °C, 30 min); gelatin drying: 40–42 °C. | |
| [45]. Collagen tissue: camel bone | 8.5–25.3 |
| Separation of non-collagenous matter: H2O (22 °C); demineralization: 1.5–6.0% HCl (22 °C, 24–120 h); filtration; washing with H2O; drying: 50 °C, 24 h; conditioning: 6.0% HCl (22 °C, 72 h); washing and neutralization: H2O (18 °C); gelatin extraction: 40–80 °C, 0.5–3.5 h, pH 1–7; gelatin freeze drying. | |
| [46]. Collagen tissues: tuna, shark and rohu skins | 11.3–19.7 |
| Washing with H2O (18 °C); separation of non-collagenous matter: 0.1 mol/L NaOH; washing and neutralization: H2O; conditioning: 0.2 mol/L CH3COOH (4 °C, 24 h); washing (neutralization): H2O; gelatin extraction: 45 °C, 12 h; fat separation; gelatin freeze drying. | |
| [47]. Collagen tissue: bovine heart | |
| Separation of non-collagenous matter: 0.5 mol/L NaOH, 22 °C, 30 min; neutralization: HCl; defatting: 10% butylalcohol, 22 °C; 1st gelatin extraction: H2O, 80 °C, 4–6 h; gelatin freeze drying (–60 °C). Conditioning: 0.5 mol/L CH3COOH + enzyme (100–200 mg/1 g of tissue): 22 °C, 24 h; neutralization: NaOH; 2nd gelatin extraction: H2O, 80 °C, 2 h; gelatin freeze drying (–60 °C). | 7.0–11.0 |
| 66.0–85.0 | |
| [8]. Collagen tissue: duck skin | 11.7–44.0 |
| Separation of non-collagenous matter: H2O (22 °C); conditioning: 0.1 mol/L HCl (18 °C, 24 h, pH 1.0); washing (neutralization): H2O (18 °C, 48 h); gelatin extraction (4 different methods): H2O (60 °C, 10 min), sonification in H2O (40 kHz, 60 °C, 10 min), steam (150 °C, 10 min), microwave (2450 MHz, 200 W, 10 min); filtration; gelatin coagulation (4 °C, 12 h); fat separation; gelatin freeze drying (–40 °C). | |
| [18]. Collagen tissue: tuna skin | 11.3 |
| Separation of non-collagenous matter and pigment: H2O (40 °C, 10 min), 0.1 mol/L NaOH (22 °C, 1 h); washing with H2O (18 °C); conditioning: 0.2 mol/L CH3COOH (4 °C, 12 h); washing and neutralization: H2O (45 °C, 12 h); gelatin extraction: 45 °C, 12 h; filtration; concentration to 15% dry matter (vacuum, 45 °C); gelatin freeze drying (–25 °C). | |
| Collagen tissue: frog skin | 7.1–15.4 |
| Separation of non-collagenous matter: 0.2 mol/L NaOH (4 °C, 30 min); washing: H2O (18 °C); conditioning: 0.05 mol/L CH3COOH (25 °C, 3 h); washing and neutralization: H2O (45 °C, 12 h); gelatin extraction: 45 °C, 12 h; filtration; concentration to 15% dry matter (vacuum, 45 °C); gelatin freeze drying (–25 °C). | |
| Collagen tissue: chicken skin | 2.2 |
| Defatting: 30% isopropylalcohol (22 °C, 2 h); separation of non-collagenous matter: 1.0% NaCl (22 °C, 30 min, pH 10.6); filtration; conditioning: 5.0% HCl (22 °C, 24 h); washing with H2O, neutralization (pH 7); gelatin extraction: 45–65 °C, 15 h; filtration; gelatin freeze drying (–25 °C). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokrejš, P.; Gál, R.; Pavlačková, J. Enzyme Conditioning of Chicken Collagen and Taguchi Design of Experiments Enhancing the Yield and Quality of Prepared Gelatins. Int. J. Mol. Sci. 2023, 24, 3654. https://doi.org/10.3390/ijms24043654
Mokrejš P, Gál R, Pavlačková J. Enzyme Conditioning of Chicken Collagen and Taguchi Design of Experiments Enhancing the Yield and Quality of Prepared Gelatins. International Journal of Molecular Sciences. 2023; 24(4):3654. https://doi.org/10.3390/ijms24043654
Chicago/Turabian StyleMokrejš, Pavel, Robert Gál, and Jana Pavlačková. 2023. "Enzyme Conditioning of Chicken Collagen and Taguchi Design of Experiments Enhancing the Yield and Quality of Prepared Gelatins" International Journal of Molecular Sciences 24, no. 4: 3654. https://doi.org/10.3390/ijms24043654
APA StyleMokrejš, P., Gál, R., & Pavlačková, J. (2023). Enzyme Conditioning of Chicken Collagen and Taguchi Design of Experiments Enhancing the Yield and Quality of Prepared Gelatins. International Journal of Molecular Sciences, 24(4), 3654. https://doi.org/10.3390/ijms24043654