Sb-Phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azastibocine Induces Perlecan Core Protein Synthesis in Cultured Vascular Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. PMTAS Enhances [35S]sulfate Incorporation into Proteoglycans in Vascular Endothelial Cells
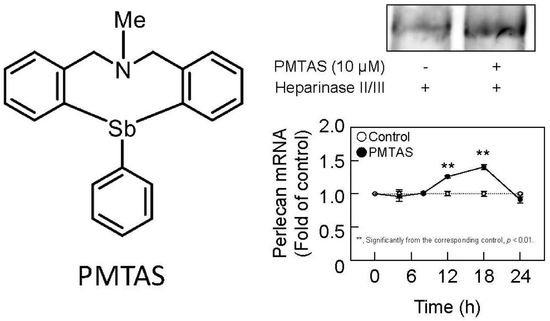
2.2. PMTAS Increases the Accumulation of Perlecan in the Conditioned Medium of Vascular Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Incorporation of [35S]sulfate into Proteoglycans
4.4. Characterization of Proteoglycans
4.5. DNA and Protein Synthesis
4.6. Analysis of Disaccharide Composition of Heparan Sulfate Chains
4.7. Analysis of Perlecan Core Protein
4.8. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujie, T.; Hara, T.; Kaji, T. Toxicology of organic-inorganic hybrid molecules: Bio-organometallics and its toxicology. J. Toxicol. Sci. 2016, 41, SP81–SP88. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, E.; Hara, T.; Fujie, T.; Yamamoto, C.; Fujiwara, Y.; Ogata, F.; Kawasaki, N.; Takita, R.; Uchiyama, M.; et al. Zn(II)2,9-dimethyl-1,10-phenanthroline stimulates cultured bovine aortic endothelial cell proliferation. RSC Adv. 2020, 10, 42327. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Matsuzaki, H.; Nakamura, T.; Yoshida, E.; Ohkubo, T.; Maruyama, H.; Yamamoto, C.; Saito, S.; Kaji, T. Cytotoxicity of zinc, copper and rhodium complexes with 1,10-phenanthroline or 2,9-dimethyl-1,10-phenanthroline in cultured vascular endothelial cells. Fundam. Toxicol. Sci. 2016, 3, 109–113. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, E.; Fujie, T.; Ogata, F.; Yamamoto, C.; Kawasaki, N.; Kaji, T. Synergistic cytotoxicity caused by forming a complex of copper and 2,9-dimethyl-1,10-phenanthroline in cultured vascular endothelial cells. J. Toxicol. Sci. 2017, 42, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Takahashi, A.; Takahashi, M.; Hara, T.; Soyama, A.; Makino, K.; Takahashi, H.; Yamamoto, C.; Kumagai, Y.; Naka, H.; et al. Transcriptional induction of cystathionine γ-lyase, a reactive sulfur-producing enzyme, by copper diethyldithiocarbamate in cultured vascular endothelial cells. Int. J. Mol. Sci. 2020, 21, 6053. [Google Scholar] [CrossRef]
- Hara, T.; Nonaka, Y.; Yasuike, S.; Kaji, T.; Yamamoto, C. Structure-activity relationship of [1,5]azastibocines in cytotoxicity to vascular endothelial cells. J. Toxicol. Sci. 2018, 43, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kohri, K.; Yoshida, E.; Yasuike, S.; Fujie, T.; Yamamoto, C.; Kaji, T. The cytotoxicity of organobismuth compounds with certain molecular structures can be diminished by replacing the bismuth atom with an antimony atom in the molecules. J. Toxicol. Sci. 2015, 40, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakano, S.; Kitamura, Y.; Yamamoto, C.; Yasuike, S.; Kaji, T. Intracellular accumulation-independent cytotoxicity of pentavalent organoantimony compounds in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 845–848. [Google Scholar] [CrossRef]
- Fujie, T.; Yamamoto, T.; Yamamoto, C.; Kaji, T. Bis(1,4-dihydro-2-methyl-1-phenyl-4-thioxo-3-pyridiolato)zinc(II) exhibits strong cytotoxicity and a high intracellular accumulation in cultured vascular endothelial cells. J. Toxicol. Sci. 2019, 44, 113–120. [Google Scholar] [CrossRef]
- Ruoslahti, E. Structure and biology of proteoglycans. Annu. Rev. Cell Biol. 1988, 4, 229–255. [Google Scholar] [CrossRef] [PubMed]
- de Agostini, A.; Watkins, S.; Slayter, H.; Youssoufian, H.; Rosenberg, R. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: Antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 1990, 111, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T. Targeted gene disruption of natural anticoagulant proteins in mice. Int. J. Hematol. 2002, 76 (Suppl. 2), 36–39. [Google Scholar] [CrossRef]
- Lin, M.; Lin, C.; Li, C.; Sun, D.; Wang, L.; Hsing, C. Anesthetic propofol overdose causes vascular hyperpermeability by reducing endothelial glycocalyx and ATP production. Int. J. Mol. Sci. 2015, 16, 12092–12107. [Google Scholar] [CrossRef] [PubMed]
- Camejo, G. The interaction of lipids and lipoproteins with the intercellular matrix of arterial tissue: Its possible role in atherogenesis. Adv. Lipid. Res. 1982, 19, 1–53. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. A role for proteoglycans in vascular disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar] [CrossRef] [PubMed]
- Saku, T.; Furthmayr, H. Characterization of the major heparan sulfate proteoglycan secreted by bovine aortic endothelial cells in culture. Homology to the large molecular weight molecule of basement membranes. J. Biol. Chem. 1989, 264, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shworak, N.; Rosenberg, R. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J. Biol. Chem. 1992, 267, 4870–4877. [Google Scholar] [CrossRef]
- Järveläinen, H.; Kinsella, M.; Wight, T.; Sandell, L. Differential expression of small chondroitin/dermatan sulfate proteoglycans, PG-I/biglycan and PG-II/decorin, by vascular smooth muscle and endothelial cells in culture. J. Biol. Chem. 1991, 266, 23274–23281. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; Cassiman, J.; Van den Berghe, H.; Vermylen, J.; David, G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 1992, 267, 20435–20443. [Google Scholar] [CrossRef]
- Gajdusek, C.M.; Carbon, S. Injury-induced release of basic fibroblast growth factor from bovine aortic endothelium. J. Cell. Physiol. 1989, 139, 570–579. [Google Scholar] [CrossRef]
- Aviezer, D.; Hecht, D.; Safran, M.; Eisinger, M.; David, G.; Yayon, A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 1994, 79, 1005–1013. [Google Scholar] [CrossRef]
- Hara, T.; Kojima, T.; Matsuzaki, H.; Nakamura, T.; Yoshida, E.; Fujiwara, Y.; Yamamoto, C.; Saito S, S.; Kaji, T. Induction of syndecan-4 by organic–inorganic hybrid molecules with a 1,10-phenanthroline structure in cultured vascular endothelial cells. Int. J. Mol. Sci. 2017, 18, 352. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Tatsuishi, H.; Banno, T.; Fujie, T.; Yamamoto, C.; Naka, H.; Kaji, T. Copper(II) bis(diethyldithiocarbamate) induces the expression of syndecan-4, a transmembrane heparan sulfate proteoglycan, via p38 MAPK activation in vascular endothelial cells. Int. J. Mol. Sci. 2018, 19, 3302. [Google Scholar] [CrossRef]
- Hara, T.; Sakamaki, S.; Ikeda, A.; Nakamura, T.; Yamamoto, C.; Kaji, T. Cell density-dependent modulation of perlecan synthesis by dichloro(2,9-dimethyl-1,10-phenanthroline)zinc(II) in vascular endothelial cells. J. Toxicol. Sci. 2020, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T.; Yamada, A.; Miyajima, S.; Yamamoto, C.; Fujiwara, Y.; Wight, T.; Kinsella, M. Cell density-dependent regulation of proteoglycan synthesis by transforming growth factor-β1 in cultured bovine aortic endothelial cells. J. Biol. Chem. 2000, 275, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yabushita, S.; Yamamoto, C.; Kaji, T. Cell density-dependent fibroblast growth factor-2 signaling regulates syndecan-4 expression in cultured vascular endothelial cells. Int. J. Mol. Sci. 2020, 21, 3698. [Google Scholar] [CrossRef]
- Hara, T.; Wakata, T.; Fujiwara, Y.; Yamamoto, C.; Kaji, T. Induction of versican V0 variant synthesis by a thrombin receptor agonist peptide in cultured human coronary smooth muscle cells. BPB Rep. 2019, 2, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kaji, T.; Yamamoto, C.; Oh-i, M.; Fujiwara, Y.; Yamazaki, Y.; Morita, T.; Plaas, A.; Wight, T. The vascular endothelial growth factor VEGF165 induces perlecan synthesis via VEGF receptor-2 in cultured human brain microvascular endothelial cells. Biochim. Biophys. Acta 2006, 1760, 1465–1474. [Google Scholar] [CrossRef]
- Saikia, P.; Thangavadivel, S.; Medeiros, C.; Lassance, L.; de Oliveira, R.; Wilson, S. IL-1 and TGF-β modulation of epithelial basement membrane components perlecan and nidogen production by corneal stromal cells. Invest. Ophthalmol. Vis. Sci. 2018, 59, 5589–5598. [Google Scholar] [CrossRef] [Green Version]
- de Yébenes, E.; Ho, A.; Damani, T.; Fillit, H.; Blum, M. Regulation of the heparan sulfate proteoglycan, perlecan, by injury and interleukin-1alpha. J. Neurochem. 1999, 73, 812–820. [Google Scholar] [CrossRef]
- Oohira, A.; Wight, T.; Bornstein, P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J. Biol. Chem. 1983, 258, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Leone, C.W.; Marchildon, G.; Marcum, J.; Rosenberg, R. Isolation and characterization of heparin sulfate proteoglycans produced by cloned rat microvascular endothelial cells. J. Biol. Chem. 1992, 267, 4859–4869. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Murakami, M.; Yoshida, E.; Yasuike, S.; Kimura, T.; Fujiwara, Y.; Yamamoto, C.; Kaji, T. Transcriptional induction of metallothionein by tris(pentafluorophenyl)stibane in cultured bovine aortic endothelial cells. Int. J. Mol. Sci. 2016, 17, 1381. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Takenaka, F.; Yoshida, E.; Yasuike, S.; Fujiwara, Y.; Shinkai, Y.; Kumagai, Y.; Yamamoto, C.; Kaji, T. Possible mechanisms underlying transcriptional induction of metallothionein isoforms by tris(pentafluorophenyl)stibane, tris(pentafluorophenyl)arsane, and tris(pentafluorophenyl)phosphane in cultured bovine aortic endothelial cells. J. Toxicol. Sci. 2019, 44, 327–333. [Google Scholar] [CrossRef]
- Muranaka, A.; Yasuike, S.; Liu, C.; Kurita, J.; Kakusawa, N.; Tsuchiya, T.; Okuda, M.; Kobayashi, N.; Matsumoto, Y.; Yoshida, K.; et al. Effect of periodic replacement of the heteroatom on the spectroscopic properties of indole and benzofuran derivatives. J. Phys. Chem. A 2009, 113, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Fujie, T.; Segawa, Y.; Yoshida, E.; Kimura, T.; Fujiwara, Y.; Yamamoto, C.; Satoh, M.; Naka, H.; Kaji, T. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J. Toxicol. Sci. 2016, 41, 225–232. [Google Scholar] [CrossRef]
- Wasteson, A.; Uthne, K.; Westermark, B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem. J. 1973, 136, 1069–1074. [Google Scholar] [CrossRef]
- Kissane, J.; Robins, E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J. Biol. Chem. 1958, 233, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yoshida, E.; Fujiwara, Y.; Yamamoto, C.; Kaji, T. Transforming growth factor-β1 modulates the expression of syndecan-4 in cultured vascular endothelial cells in a biphasic manner. J. Cell. Biochem. 2017, 118, 2009–2017. [Google Scholar] [CrossRef] [Green Version]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, T.; Konishi, T.; Yasuike, S.; Fujiwara, Y.; Yamamoto, C.; Kaji, T. Sb-Phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azastibocine Induces Perlecan Core Protein Synthesis in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 3656. https://doi.org/10.3390/ijms24043656
Hara T, Konishi T, Yasuike S, Fujiwara Y, Yamamoto C, Kaji T. Sb-Phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azastibocine Induces Perlecan Core Protein Synthesis in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(4):3656. https://doi.org/10.3390/ijms24043656
Chicago/Turabian StyleHara, Takato, Tomoko Konishi, Shuji Yasuike, Yasuyuki Fujiwara, Chika Yamamoto, and Toshiyuki Kaji. 2023. "Sb-Phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azastibocine Induces Perlecan Core Protein Synthesis in Cultured Vascular Endothelial Cells" International Journal of Molecular Sciences 24, no. 4: 3656. https://doi.org/10.3390/ijms24043656
APA StyleHara, T., Konishi, T., Yasuike, S., Fujiwara, Y., Yamamoto, C., & Kaji, T. (2023). Sb-Phenyl-N-methyl-5,6,7,12-tetrahydrodibenz[c,f][1,5]azastibocine Induces Perlecan Core Protein Synthesis in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences, 24(4), 3656. https://doi.org/10.3390/ijms24043656