Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients
Abstract
:1. Introduction
2. Results
2.1. Study Design and Subject Characteristics
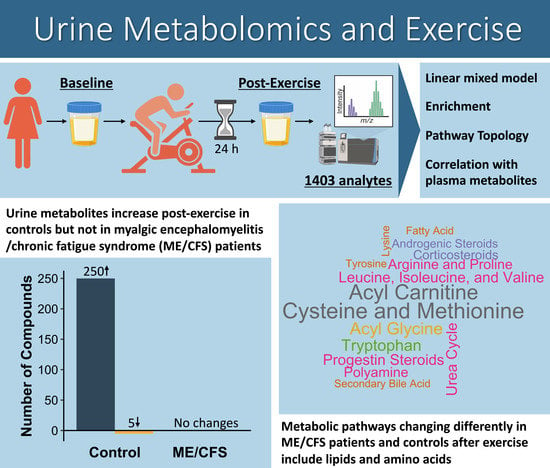
2.2. Many Differences in the Urine Metabolomes of ME/CFS Patients and Controls Emerge through Analysis of Changes between Pre- and Post-Exercise Samples
2.3. Two Approaches to Enrichment Analysis Reveal Metabolic Subpathways with the Most Significant Changes
2.4. A Pathway Topology Analysis Highlights Altered Carbohydrates and Amino Acid Metabolism as a Result of Exercise
2.5. Acyl Glycines Have Lower Concentrations in the Urine of ME/CFS Patients Compared to Controls 24 Hours Post-Exercise
2.6. Metabolites That Are Changing Differently during Exercise Recovery in ME/CFS Patients vs. Controls Are Predominantly Amino Acids and Lipids
2.7. The Same Metabolites in Urine and Plasma Are Highly Correlated
2.8. Probing Compounds with Correlations between Urine and Plasma That Are Different in ME/CFS Patients and Controls
3. Discussion
3.1. Comparison to Previous Urine Metabolomics Studies in ME/CFS Patients
3.2. The Post-Exercise Increase in Urinary Metabolite Levels in Sedentary Controls Is Consistent with Previous Studies
3.3. Differences between Sedentary Controls and ME/CFS Patients in the Lipid Superpathway
3.4. Differences between Sedentary Controls and ME/CFS Patients in the Amino Acid Superpathway
3.5. Limitations
4. Materials and Methods
4.1. Study Subjects
4.2. Cardiopulmonary Exercise Testing and Urine Sample Collection
4.3. Metabolomics Assay
4.4. Data Processing
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jason, L.A.; Mirin, A.A. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue Biomed. Health Behav. 2021, 9, 9–13. [Google Scholar] [CrossRef]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey. PLoS ONE 2018, 13, e0197811. [Google Scholar] [CrossRef]
- Stussman, B.; Williams, A.; Snow, J.; Gavin, A.; Scott, R.; Nath, A.; Walitt, B. Characterization of Post-exertional Malaise in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Neurol. 2020, 11, 1025. [Google Scholar] [CrossRef]
- Stevens, S.; Snell, C.; Stevens, J.; Keller, B.; VanNess, J.M. Cardiopulmonary Exercise Test Methodology for Assessing Exertion Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatr. 2018, 6, 242. [Google Scholar] [CrossRef]
- Vanness, J.M.; Snell, C.R.; Stevens, S.R. Diminished Cardiopulmonary Capacity During Post-Exertional Malaise. J. Chronic Fatigue Syndr. 2007, 14, 77–85. [Google Scholar] [CrossRef]
- Keller, B.A.; Pryor, J.L.; Giloteaux, L. Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO(2)peak indicates functional impairment. J. Transl. Med. 2014, 12, 104. [Google Scholar] [CrossRef]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2019, 9, 80. [Google Scholar] [CrossRef]
- Huth, T.K.; Eaton-Fitch, N.; Staines, D.; Marshall-Gradisnik, S. A systematic review of metabolomic dysregulation in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis/Systemic Exertion Intolerance Disease (CFS/ME/SEID). J. Transl. Med. 2020, 18, 198. [Google Scholar] [CrossRef]
- Germain, A.; Barupal, D.K.; Levine, S.M.; Hanson, M.R. Comprehensive Circulatory Metabolomics in ME/CFS Reveals Disrupted Metabolism of Acyl Lipids and Steroids. Metabolites 2020, 10, 34. [Google Scholar] [CrossRef]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol. Biosyst. 2017, 13, 371–379. [Google Scholar] [CrossRef]
- Germain, A.; Ruppert, D.; Levine, S.M.; Hanson, M.R. Prospective Biomarkers from Plasma Metabolomics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Implicate Redox Imbalance in Disease Symptomatology. Metabolites 2018, 8, 90. [Google Scholar] [CrossRef]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Iwai, K.; Fukuda, S.; et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci. Rep. 2016, 6, 34990. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Sheedy, J.R.; Buttfield, I.; Butt, H.L.; Gooley, P.R. NMR metabolic profiling of serum identifies amino acid disturbances in chronic fatigue syndrome. Clin. Chim. Acta 2012, 413, 1525–1531. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. The association of fecal microbiota and fecal, blood serum and urine metabolites in myalgic encephalomyelitis/chronic fatigue syndrome. Metabolomics 2016, 13, 8. [Google Scholar] [CrossRef]
- Hoel, F.; Hoel, A.; Pettersen, I.K.; Rekeland, I.G.; Risa, K.; Alme, K.; Sorland, K.; Fossa, A.; Lien, K.; Herder, I.; et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2021, 6, e149217. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Barupal, D.K.; Lee, B.; Che, X.; Williams, B.L.; Kahn, E.J.R.; Ukaigwe, J.E.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; et al. Insights into myalgic encephalomyelitis/chronic fatigue syndrome phenotypes through comprehensive metabolomics. Sci. Rep. 2018, 8, 10056. [Google Scholar] [CrossRef]
- McGregor, N.R.; Armstrong, C.W.; Lewis, D.P.; Gooley, P.R. Post-Exertional Malaise Is Associated with Hypermetabolism, Hypoacetylation and Purine Metabolism Deregulation in ME/CFS Cases. Diagnostics 2019, 9, 70. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 2015, 11, 1626–1639. [Google Scholar] [CrossRef]
- Fluge, O.; Mella, O.; Bruland, O.; Risa, K.; Dyrstad, S.E.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Rosland, G.V.; Fossa, A.; et al. Metabolic profiling indicates impaired pyruvate dehydrogenase function in myalgic encephalopathy/chronic fatigue syndrome. JCI Insight 2016, 1, e89376. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic features of chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E5472–E5480. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Cooper, E.; Amjad, S.; Goodwin, C.S.; Barron, J.L.; Chalmers, R.A. Urinary and plasma organic acids and amino acids in chronic fatigue syndrome. Clin. Chim. Acta 2005, 361, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Niblett, S.H.; King, K.E.; Dunstan, R.H.; Clifton-Bligh, P.; Hoskin, L.A.; Roberts, T.K.; Fulcher, G.R.; McGregor, N.R.; Dunsmore, J.C.; Butt, H.L.; et al. Hematologic and urinary excretion anomalies in patients with chronic fatigue syndrome. Exp. Biol. Med. 2007, 232, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Giloteaux, L.; Moore, G.E.; Levine, S.M.; Chia, J.K.; Keller, B.A.; Stevens, J.; Franconi, C.J.; Mao, X.; Shungu, D.C.; et al. Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2022, 7, e157621. [Google Scholar] [CrossRef]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome. Understanding, Treating, and Living with CFIDS; Addison-Wesley Publishing Company: Reading, MA, USA, 1994. [Google Scholar]
- Yang, J.; Zhao, X.; Lu, X.; Lin, X.; Xu, G. A data preprocessing strategy for metabolomics to reduce the mask effect in data analysis. Front. Mol. Biosci. 2015, 2, 4. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fan, S.; Fiehn, O. Integrating bioinformatics approaches for a comprehensive interpretation of metabolomics datasets. Curr. Opin. Biotechnol. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef]
- Goeman, J.J.; van de Geer, S.A.; de Kort, F.; van Houwelingen, H.C. A global test for groups of genes: Testing association with a clinical outcome. Bioinformatics 2004, 20, 93–99. [Google Scholar] [CrossRef]
- Rosato, A.; Tenori, L.; Cascante, M.; De Atauri Carulla, P.R.; Martins Dos Santos, V.A.P.; Saccenti, E. From correlation to causation: Analysis of metabolomics data using systems biology approaches. Metabolomics 2018, 14, 37. [Google Scholar] [CrossRef]
- Cardounel, A.J.; Cui, H.; Samouilov, A.; Johnson, W.; Kearns, P.; Tsai, A.L.; Berka, V.; Zweier, J.L. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J. Biol. Chem. 2007, 282, 879–887. [Google Scholar] [CrossRef]
- Chandrasekharan, U.M.; Wang, Z.; Wu, Y.; Wilson Tang, W.H.; Hazen, S.L.; Wang, S.; Elaine Husni, M. Elevated levels of plasma symmetric dimethylarginine and increased arginase activity as potential indicators of cardiovascular comorbidity in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 123. [Google Scholar] [CrossRef]
- Siroen, M.P.; Teerlink, T.; Nijveldt, R.J.; Prins, H.A.; Richir, M.C.; van Leeuwen, P.A. The clinical significance of asymmetric dimethylarginine. Annu. Rev. Nutr. 2006, 26, 203–228. [Google Scholar] [CrossRef]
- Bertinat, R.; Villalobos-Labra, R.; Hofmann, L.; Blauensteiner, J.; Sepulveda, N.; Westermeier, F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vasc. Pharmacol. 2022, 143, 106953. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef]
- Fernández-García, J.C.; Martínez-Sánchez, M.A.; Bernal-López, M.R.; Muñoz-Garach, A.; Martínez-González, M.A.; Fitó, M.; Salas-Salvadó, J.; Tinahones, F.J.; Ramos-Molina, B. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on the serum polyamine metabolome in individuals at high cardiovascular disease risk: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 975–982. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, H.; Xue, G.; Zhang, L.; Zhang, W.; Wang, L.; Lu, F.; Li, H.; Bai, S.; Lin, Y.; et al. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid. Med. Cell. Longev. 2014, 2014, 457429. [Google Scholar] [CrossRef]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.K.; Kohnke, R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006, 136, 269S–273S. [Google Scholar] [CrossRef]
- Newsholme, P.; Stenson, L.; Sulvucci, M.; Sumayao, R.; Krause, M. 1.02-Amino Acid Metabolism. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, ON, Canada, 2011; pp. 3–14. [Google Scholar] [CrossRef]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Mineralocorticoids. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; p. 1. [Google Scholar] [CrossRef]
- Kavyani, B.; Lidbury, B.A.; Schloeffel, R.; Fisher, P.R.; Missailidis, D.; Annesley, S.J.; Dehhaghi, M.; Heng, B.; Guillemin, G.J. Could the kynurenine pathway be the key missing piece of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) complex puzzle? Cell. Mol. Life Sci. 2022, 79, 412. [Google Scholar] [CrossRef] [PubMed]
- Kossman, D.A.; Williams, N.I.; Domchek, S.M.; Kurzer, M.S.; Stopfer, J.E.; Schmitz, K.H. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J. Appl. Physiol. 2011, 111, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Van Heest, J.; Demers, L.M.; Lasley, B.L. Luteal Phase Deficiency in Recreational Runners: Evidence for a Hypometabolic State. J. Clin. Endocrinol. Metab. 2003, 88, 337–346. [Google Scholar] [CrossRef]
- Mukherjee, K.; Edgett, B.A.; Burrows, H.W.; Castro, C.; Griffin, J.L.; Schwertani, A.G.; Gurd, B.J.; Funk, C.D. Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50–60 year old masters athletes. PLoS ONE 2014, 9, e92031. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmuller, G.; Schonfelder, M.; Romisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Kistner, S.; Rist, M.J.; Doring, M.; Dorr, C.; Neumann, R.; Hartel, S.; Bub, A. An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans-Results of the KarMeN Study. Metabolites 2020, 10, 212. [Google Scholar] [CrossRef]
- Kuratsune, H.; Yamaguti, K.; Takahashi, M.; Misaki, H.; Tagawa, S.; Kitani, T. Acylcarnitine deficiency in chronic fatigue syndrome. Clin. Infect. Dis. 1994, 18 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- Jones, M.G.; Goodwin, C.S.; Amjad, S.; Chalmers, R.A. Plasma and urinary carnitine and acylcarnitines in chronic fatigue syndrome. Clin. Chim. Acta 2005, 360, 173–177. [Google Scholar] [CrossRef]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef]
- Costa, C.G.; Guerand, W.S.; Struys, E.A.; Holwerda, U.; ten Brink, H.J.; Tavares de Almeida, I.; Duran, M.; Jakobs, C. Quantitative analysis of urinary acylglycines for the diagnosis of beta-oxidation defects using GC-NCI-MS. J. Pharm. Biomed. Anal. 2000, 21, 1215–1224. [Google Scholar] [CrossRef]
- Maya, J.; Leddy, S.M.; Gottschalk, C.G.; Peterson, D.L.; Hanson, M.R. Altered Fatty Acid Oxidation in Lymphocyte Populations of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2023, 24, 2010. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef]
- Chen, S.; Minegishi, Y.; Hasumura, T.; Shimotoyodome, A.; Ota, N. Involvement of ammonia metabolism in the improvement of endurance performance by tea catechins in mice. Sci. Rep. 2020, 10, 6065. [Google Scholar] [CrossRef]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef]
- Nkiliza, A.; Parks, M.; Cseresznye, A.; Oberlin, S.; Evans, J.E.; Darcey, T.; Aenlle, K.; Niedospial, D.; Mullan, M.; Crawford, F.; et al. Sex-specific plasma lipid profiles of ME/CFS patients and their association with pain, fatigue, and cognitive symptoms. J. Transl. Med. 2021, 19, 370. [Google Scholar] [CrossRef]
- O’Neal, A.J.; Glass, K.A.; Emig, C.J.; Vitug, A.A.; Henry, S.J.; Shungu, D.C.; Mao, X.; Levine, S.M.; Hanson, M.R. Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proteomes 2022, 10, 21. [Google Scholar] [CrossRef]
- Analysis of Post-Exertional Malaise Using a Two-Day CPET in People with ME/CFS. Available online: https://clinicaltrials.gov/ct2/show/NCT04026425 (accessed on 14 December 2022).
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Ford, L.; Kennedy, A.D.; Goodman, K.D.; Pappan, K.L.; Evans, A.M.; Miller, L.A.D.; Wulff, J.E.; Wiggs, B.R.; Lennon, J.J.; Elsea, S.; et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J. Appl. Lab. Med. 2020, 5, 342–356. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Durbin, B.P.; Hardin, J.S.; Hawkins, D.M.; Rocke, D.M. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics 2002, 18 (Suppl. 1), S105–S110. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means, 1.8.2; R Package. 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 31 January 2022).
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling, Version 1.16.0; R Package. 2022. Available online: https://bioconductor.org/packages/release/bioc/html/EnhancedVolcano.html (accessed on 11 November 2022).
- Smirnov, N.V. On the estimation of the discrepancy between empirical curves of distribution for two independent samples. Bull. Math. Univ. Moscou 1939, 2, 3–14. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps, 1.0.12; R Package. 2019. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 16 February 2022).










| ME/CFS | Controls | ||||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p-Value | |
| Age (years) | 51.5 | 5.3 | 52.5 | 6.5 | 0.96 |
| BMI | 24 | 10.9 | 33.3 | 8.2 | 0.03 * |
| Bell disability scale | 30 | 15 | 90 | 22.5 | 0.0004 * |
| SF-36 physical component summary (PCS) | 25.6 | 7 | 54.5 | 7.1 | 0.00005 * |
| SF-36 mental component summary (MCS) | 48 | 4.6 | 57 | 6.2 | 0.07 |
| Multidimensional fatigue inventory (MFI) | 83 | 10.3 | NA | NA | NA |
| ME/CFS duration (years) | 7.5 | 7.3 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glass, K.A.; Germain, A.; Huang, Y.V.; Hanson, M.R. Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients. Int. J. Mol. Sci. 2023, 24, 3685. https://doi.org/10.3390/ijms24043685
Glass KA, Germain A, Huang YV, Hanson MR. Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients. International Journal of Molecular Sciences. 2023; 24(4):3685. https://doi.org/10.3390/ijms24043685
Chicago/Turabian StyleGlass, Katherine A., Arnaud Germain, Yuhsin V. Huang, and Maureen R. Hanson. 2023. "Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients" International Journal of Molecular Sciences 24, no. 4: 3685. https://doi.org/10.3390/ijms24043685
APA StyleGlass, K. A., Germain, A., Huang, Y. V., & Hanson, M. R. (2023). Urine Metabolomics Exposes Anomalous Recovery after Maximal Exertion in Female ME/CFS Patients. International Journal of Molecular Sciences, 24(4), 3685. https://doi.org/10.3390/ijms24043685