Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity
Abstract
:1. Introduction
2. Results
2.1. Melatonin Positively Modulates Cr Stress Resistance in Maize
2.2. Melatonin Cannot Influence the Cr Uptake in Maize
2.3. Melatonin and Cr Treatments Induce Transcriptome Reprogramming in Maize
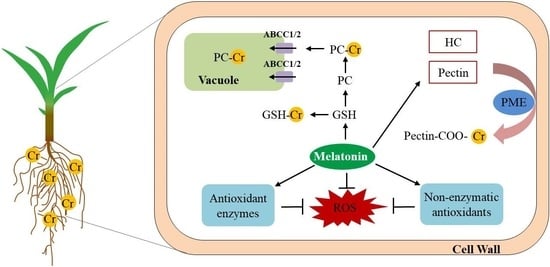
2.4. Melatonin Enhances Cr Accumulation in the Cell Walls in Maize
2.5. Melatonin Increases the Glutathione and Phytochelatin Contents in Maize
2.6. Melatonin Increases Antioxidant Capacity in Maize
2.7. Melatonin Increases Endogenous Melatonin Content in Maize
2.8. Modulation of Melatonin Content in Arabidopsis Confers Enhanced Cr Stress Tolerance
3. Discussion
3.1. Melatonin Enhances Cr Binding Capacity of Cell Walls in Maize
3.2. Melatonin Promotes Cr Chelation in Maize
3.3. Melatonin Increases Antioxidant Capacity in Maize
4. Materials and Methods
4.1. Experimental Design
4.2. Determination of Dry Weight, Root Vigor, Leaf Area, Chlorophyll Content, and Photosynthetic Rate
4.3. Determination of Cr Content
4.4. Transcriptome Sequencing and qRT-PCR Analyses
4.5. Metabolite Content Assays
4.6. Determination of Enzyme Activity
4.7. Determination of Melatonin Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of hexavalent chromium: Current trends and promising perspectives. J. Environ. Manag. 2021, 279, 111547. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Ethylene needs endogenous hydrogen sulfide for alleviating hexavalent chromium stress in Vigna mungo L. and Vigna radiata L. Environ. Pollut. 2021, 290, 117968. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Y.; Khan, A.R.; Wang, J.; Zhou, W. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Bioch. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic acid underpins silicon in ameliorating chromium toxicity in rice by modulating antioxidant defense, ion homeostasis and cellular ultrastructure. Plant Physiol. Bioch. 2021, 166, 1001–1013. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. 2022, 29, 70686–70705. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Liang, J.; Chen, X.; Ye, J.; Liu, Y.; Liu, Y.; Wei, Y. Remediation of hexavalent chromium in contaminated soil using amorphous iron pyrite: Effect on leachability, bioaccessibility, phytotoxicity and long-term stability. Environ. Pollut. 2020, 264, 114804. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Zhang, N.; Feng, L.; Ma, C.; Wang, Y.; Yang, Z.P.; Zhao, J. Melatonin alleviates aluminum-induced growth inhibition by modulating carbon and nitrogen metabolism, and reestablishing redox homeostasis in Zea mays L. J. Hazard. Mater. 2022, 423, 127159. [Google Scholar] [CrossRef]
- Liu, X.X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Kharbech, O.; Sakouhi, L.; Mahjoubi, Y.; Massoud, M.B.; Debez, A.; Zribi, O.T.; Djebali, W.; Chaoui, A.; Mur, L.A.J. Nitric oxide donor, sodium nitroprusside modulates hydrogen sulfide metabolism and cysteine homeostasis to aid the alleviation of chromium toxicity in maize seedlings (Zea mays L.). J. Hazard. Mater. 2022, 424, 127302. [Google Scholar] [CrossRef]
- Colzi, I.; Arnetoli, M.; Gallo, A.; Doumett, S.; Del Bubba, M.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Copper tolerance strategies involving the root cell wall pectins in Silene paradoxa L. Environ. Exp. Bot. 2012, 78, 91–98. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, S.; Zhang, K.; Shi, X.; Lian, C.; Zhang, H.; Hu, Z.; Shen, Z. OsWAK11, a rice wall-associated kinase, regulates Cu detoxification by alteration the immobilization of Cu in cell walls. Environ. Exp. Bot. 2018, 150, 99–105. [Google Scholar] [CrossRef]
- Greco, M.; Sáez, C.A.; Contreras, R.A.; Rodríguez-Rojas, F.; Bitonti, M.B.; Brown, M.T. Cadmium and/or copper excess induce interdependent metal accumulation, DNA methylation, induction of metal chelators and antioxidant defences in the seagrass Zostera marina. Chemosphere 2019, 224, 111–119. [Google Scholar] [CrossRef]
- Navarrete, A.; González, A.; Gómez, M.; Contreras, R.A.; Díaz, P.; Lobos, G.; Moenne, A. Copper excess detoxification is mediated by a coordinated and complementary induction of glutathione, phytochelatins and metallothioneins in the green seaweed Ulva compressa. Plant Physiol. Bioch. 2019, 135, 423–431. [Google Scholar] [CrossRef]
- He, G.; Tian, W.; Qin, L.; Meng, L.; Wu, D.; Huang, Y.; Li, D.; Zhao, D.; He, T. Identification of novel heavy metal detoxification proteins in Solanum tuberosum: Insights to improve food security protection from metal ion stress. Sci. Total Environ. 2021, 779, 146197. [Google Scholar] [CrossRef]
- Yadav, P.; Kaur, R.; Kanwar, M.K.; Sharma, A.; Verma, V.; Sirhindi, G.; Bhardwaj, R. Castasterone confers copper stress tolerance by regulating antioxidant enzyme responses, antioxidants, and amino acid balance in B. juncea seedlings. Ecotox. Environ. Saf. 2018, 147, 725–734. [Google Scholar] [CrossRef]
- Hossain, M.S.; Abdelrahman, M.; Tran, C.D.; Nguyen, K.H.; Chu, H.D.; Watanabe, Y.; Hasanuzzaman, M.; Mohsin, S.M.; Fujita, M.; Tran, L.S.P. Insights into acetate-mediated copper homeostasis and antioxidant defense in lentil under excessive copper stress. Environ. Pollut. 2020, 258, 113544. [Google Scholar] [CrossRef]
- Del Pozo, T.; Cambiazo, V.; González, M. Gene expression profiling analysis of copper homeostasis in Arabidopsis thaliana. Biochem. Bioph. Res. Co. 2010, 393, 248–252. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Hoque, M.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An unexpected molecule with amazing performances in plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Ren, W.; Chen, L.; Xie, Z.; Peng, X. Combined transcriptome and metabolome analysis revealed pathways involved in improved salt tolerance of Gossypium hirsutum L. seedlings in response to exogenous melatonin application. BMC Plant Biol. 2022, 22, 552. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, J.; Tian, X.; Yang, Z.; Fang, W. Nitric oxide mediates melatonin-induced isoflavone accumulation and growth improvement in germinating soybeans under NaCl stress. J. Plant Physiol. 2022, 279, 153855. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Y.; Sun, H.; Bao, Q.; Huang, Y. Melatonin alleviates arsenite toxicity by decreasing the arsenic accumulation in cell protoplasts and increasing the antioxidant capacity in rice. Chemosphere 2023, 312, 137292. [Google Scholar] [CrossRef]
- Jan, R.; Asif, S.; Asaf, S.; Lubna; Du, X.; Park, J.; Nari, K.; Bhatta, D.; Lee, I.; Kim, K. Melatonin alleviates arsenic (As) toxicity in rice plants via modulating antioxidant defense system and secondary metabolites and reducing oxidative stress. Environ. Pollut. 2022, 318, 120868. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Amri, S.M. Ameliorative roles of melatonin and/or zeolite on chromium-induced leaf senescence in marjoram plants by activating antioxidant defense, osmolyte accumulation, and ultrastructural modification. Ind. Crop. Prod. 2019, 142, 111823. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotox. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Qi, C.D.; Li, S.; Wang, Z.; Wang, X.; Wang, J.; Ren, S.X.; Li, X.S.; Zhang, N.; Guo, Y.D. Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol. 2019, 60, 562–574. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, J. Ameliorative effects of Lanthanum(III) on Copper(II) stressed rice (Oryza sativa) and its molecular mechanism revealed by transcriptome profiling. Plant Physiol. Bioch. 2020, 152, 184–193. [Google Scholar] [CrossRef]
- Lwalaba, J.L.W.; Zvobgo, G.; Gai, Y.P.; Issaka, J.H.; Mwamba, T.M.; Louis, L.T.; Fu, L.B.; Nazir, M.M.; Kirika, B.A.; Tshibangu, A.K.; et al. Transcriptome analysis reveals the tolerant mechanisms to cobalt and copper in barley. Ecotox. Environ. Saf. 2021, 209, 111761. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef]
- Hou, Q.; Wan, X. Epigenome and Epitranscriptome: Potential Resources for Crop Improvement. Int. J. Mol. Sci. 2021, 22, 12912. [Google Scholar] [CrossRef]
- Liu, P.; Jin, Z.; Dai, C.; Guo, L.; Cui, X.; Yang, Y. Potassium enhances cadmium resistance ability of Panax notoginseng by brassinolide signaling pathway-regulated cell wall pectin metabolism. Ecotox. Environ. Saf. 2021, 227, 112906. [Google Scholar] [CrossRef]
- Yuan, Y.; Imtiaz, M.; Rizwan, M.; Dai, Z.; Hossain, M.M.; Zhang, Y.; Huang, H.L.; Tu, S. The role and its transcriptome mechanisms of cell wall polysaccharides in vanadium detoxication of rice. J. Hazard. Mater. 2022, 425, 127966. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, X.; Zhu, Z.; Zhou, K. Sodium hydrosulfide mitigates cadmium toxicity by promoting cadmium retention and inhibiting its translocation from roots to shoots in Brassica napus. J. Agric. Food Chem. 2019, 67, 433–440. [Google Scholar] [CrossRef]
- Sun, C.; Lv, T.; Huang, L.; Liu, X.; Jin, C.; Lin, X. Melatonin ameliorates aluminum toxicity through enhancing aluminum exclusion and reestablishing redox homeostasis in roots of wheat. J. Pineal Res. 2020, 68, e12642. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, Z.; Wang, T.; Mantri, N.; Huang, H.; Li, H.; Tao, Z.; Guo, Q. Physiological and transcriptomic analyses of cadmium stress response in Dendrobium officinale seedling. Plant Physiol. Bioch. 2020, 148, 152–165. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Upreti, S.; Azizullah, A.; Liu, B.; Khan, A.R.; Huang, L.; Wu, M.; Gan, Y. Ethylene mediates dichromate-induced inhibition of primary root growth by altering AUX1 expression and auxin accumulation in Arabidopsis thaliana. Plant Cell Environ. 2018, 41, 1453–1467. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.L.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.J.; Wang, Y.Q.; Yan, Y.R.; Mao, Q.; Ren, J.J.; Ma, R.H.; Liu, A.R.; Chen, S.C. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotox. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Hasan, M.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78. [Google Scholar] [CrossRef]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in nicotiana tabacum L. Ecotox. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Zhan, J.; Huang, H.; Yu, H.; Zhang, X.; Zheng, Z.; Wang, Y.; Liu, T.; Li, T. The combined effects of Cd and Pb enhanced metal binding by root cell walls of the phytostabilizer Athyrium wardii (Hook.). Environ. Pollut. 2020, 258, 113663. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Liu, D.; Yao, J.; Liang, G.; Song, H.; Ismail, A.; Luo, J.; Zhang, Z. Cell wall polysaccharide-mediated cadmium tolerance between two Arabidopsis thaliana ecotypes. Front. Plant Sci. 2020, 11, 473. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Lv, A.; Li, Y.; Zhou, P.; An, Y. MsPG1 alleviated aluminum-induced inhibition of root growth by decreasing aluminum accumulation and increasing porosity and extensibility of cell walls in alfalfa (Medicago sativa). Environ. Exp. Bot. 2020, 175, 104045. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef]
- Wu, X.; Tian, H.; Li, L.; Wang, X. Polyaspartic acid alleviates cadmium toxicity in rapeseed leaves by affecting cadmium translocation and cell wall fixation of cadmium. Ecotox. Environ. Saf. 2021, 224, 112685. [Google Scholar] [CrossRef]
- Wu, X.; Tian, H.; Li, L.; Guan, C.; Zhang, Z. Higher Cd-accumulating oilseed rape has stronger Cd tolerance due to stronger Cd fixation in pectin and hemicellulose and higher Cd chelation. Environ. Pollut. 2021, 285, 117218. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Qin, M.L.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef]
- Goodarzi, A.; Namdjoyan, S.; Soorki, A.A. Effects of exogenous melatonin and glutathione on zinc toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotox. Environ. Saf. 2020, 201, 110853. [Google Scholar] [CrossRef]
- Kumar, V.; Khare, T.; Shriram, V.; Wani, S.H. Plant small RNAs: The essential epigenetic regulators of gene expression for salt-stress responses and tolerance. Plant Cell Rep. 2018, 37, 61–75. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Reinfelder, J.R.; Liang, X.; Sun, C.; Liu, C.; Li, F.; Yi, J. A transcriptomic (RNA-seq) analysis of genes responsive to both cadmium and arsenic stress in rice root. Sci. Total Environ. 2019, 666, 445–460. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Khan, M.N.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard Mater. 2020, 398, 122882. [Google Scholar] [CrossRef] [PubMed]
- Kühnlenz, T.; Westphal, L.; Schmidt, H.; Scheel, D.; Clemens, S. Expression of Caenorhabditis elegans PCS in the AtPCS1-deficient Arabidopsis thaliana cad1-3 mutant separates the metal tolerance and non-host resistance functions of phytochelatin synthases. Plant Cell Environ. 2015, 38, 2239–2247. [Google Scholar] [CrossRef]
- Gasic, K.; Korban, S.S. Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 2007, 225, 1277–1285. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Ding, Y.; Wang, X.; Xu, J. Overexpression of PtPCS enhances cadmium tolerance and cadmium accumulation in tobacco. Plant Cell Tiss. Org. 2015, 121, 389–396. [Google Scholar] [CrossRef]
- Hasan, M.; Ahammed, G.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Samanta, S.; Banerjee, A.; Roychoudhury, A. Exogenous melatonin regulates endogenous phytohormone homeostasis and thiol-mediated detoxification in two indica rice cultivars under arsenic stress. Plant Cell Rep. 2021, 40, 1585–1602. [Google Scholar] [CrossRef]
- Hasan, M.; Cheng, Y.; Kanwar, M.; Chu, X.; Ahammed, G.; Qi, Z. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.; Du, J.; He, J.; Lyu, D.; Li, H. Copper accumulation, subcellular partitioning and physiological and molecular responses in relation to different copper tolerance in apple rootstocks. Tree Physiol. 2019, 39, 1215–1234. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, X.N.; Zhang, L.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard Mater. 2021, 403, 123922. [Google Scholar] [CrossRef]
- Abo Gamar, M.I.; Kisiala, A.; Emery, R.J.; Yeung, E.C.; Stone, S.L.; Qaderi, M.M. Elevated carbon dioxide decreases the adverse effects of higher temperature and drought stress by mitigating oxidative stress and improving water status in Arabidopsis thaliana. Planta 2019, 250, 1191–1214. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Hydrogen sulfide (H2S) mitigates arsenic (As)-induced toxicity in pea (Pisum sativum L.) plants by regulating osmoregulation, antioxidant defense system, ascorbate glutathione cycle and glyoxalase system. J. Plant Growth Regul. 2021, 40, 2515–2531. [Google Scholar] [CrossRef]
- Singh, S.; Husain, T.; Kushwaha, B.K.; Suhel, M.; Fatima, A.; Mishra, V.; Singh, S.K.; Bhatt, J.A.; Rai, M.; Prasad, S.M.; et al. Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J. Hazard. Mater. 2021, 409, 123686. [Google Scholar] [CrossRef]
- Bao, Q.; Bao, W.; Li, Y.; Zhang, S.; Lian, F.; Huang, Y. Silicon combined with foliar melatonin for reducing the absorption and translocation of Cd and As by Oryza sativa L. in two contaminated soils. J. Environ. Manage. 2021, 287, 112343. [Google Scholar] [CrossRef]
- Okant, M.; Kaya, C. The role of endogenous nitric oxide in melatonin-improved tolerance to lead toxicity in maize plants. Environ. Sci. Pollut. Res. 2019, 26, 11864–11874. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.H.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop. Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Q.; Liu, Y.; Cheng, P.; Cheng, H.; Liu, W.; Xing, X.; Guan, Z.; Fang, W.; Chen, S.; et al. Strigolactone represses the synthesis of melatonin, thereby inducing floral transition in Arabidopsis thaliana in an FLC-dependent manner. J. Pineal Res. 2019, 67, e12582. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. The effectiveness of grafting to improve drought tolerance in tomato. Plant Growth Regul. 2020, 91, 157–167. [Google Scholar] [CrossRef]
- Xu, D.; Xie, Y.; Li, J. Toxic effects and molecular mechanisms of sulfamethoxazole on Scenedesmus obliquus. Ecotox. Environ. Saf. 2022, 232, 113258. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, 316–322. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Bačkor, M. Zn/Pb-tolerant lichens with higher content of secondary metabolites produce less phytochelatins than specimens living in unpolluted habitats. Environ. Exp. Bot. 2011, 72, 64–70. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plantarum. 2020, 168, 289–300. [Google Scholar] [CrossRef]
- Singha, L.P.; Sinha, N.; Pandey, P. Rhizoremediation prospects of polyaromatic hydrocarbon degrading rhizobacteria, that facilitate glutathione and glutathione-S-transferase mediated stress response, and enhance growth of rice plants in pyrene contaminated soil. Ecotox. Environ. Saf. 2018, 164, 579–588. [Google Scholar] [CrossRef]
- Wojas, S.; Clemens, S.; Hennig, J.; Skłodowska, A.; Kopera, E.; Schat, H.; Bal, W.; Antosiewicz, D.M. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008, 59, 2205–2219. [Google Scholar] [CrossRef]
- Han, Y.; Zveushe, O.K.; Dong, F.Q.; Ling, Q.; Chen, Y.; Sajid, S.; Zhou, L.; De Dios, V.R. Unraveling the effects of arbuscular mycorrhizal fungi on cadmium uptake and detoxification mechanisms in perennial ryegrass (Lolium perenne). Sci. Total Environ. 2021, 798, 149222. [Google Scholar] [CrossRef]
- Qin, F.; Liu, G.; Huang, G.; Dong, T.; Liao, Y.; Xu, X. Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environ. Exp. Bot. 2018, 146, 68–76. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 3816. https://doi.org/10.3390/ijms24043816
Yang X, Ren J, Lin X, Yang Z, Deng X, Ke Q. Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. International Journal of Molecular Sciences. 2023; 24(4):3816. https://doi.org/10.3390/ijms24043816
Chicago/Turabian StyleYang, Xiaoxiao, Jianhong Ren, Xinyue Lin, Zhenping Yang, Xiping Deng, and Qingbo Ke. 2023. "Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity" International Journal of Molecular Sciences 24, no. 4: 3816. https://doi.org/10.3390/ijms24043816
APA StyleYang, X., Ren, J., Lin, X., Yang, Z., Deng, X., & Ke, Q. (2023). Melatonin Alleviates Chromium Toxicity in Maize by Modulation of Cell Wall Polysaccharides Biosynthesis, Glutathione Metabolism, and Antioxidant Capacity. International Journal of Molecular Sciences, 24(4), 3816. https://doi.org/10.3390/ijms24043816