Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices
Abstract
:1. Introduction
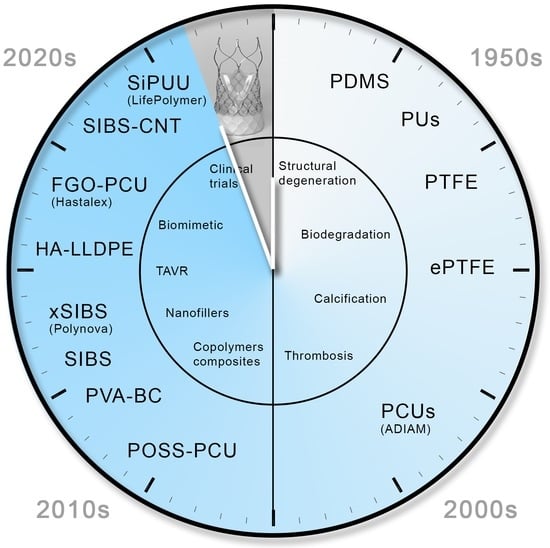
2. Historical Background
2.1. The First-Generation Polymers of Heart Valves
2.1.1. Polysiloxanes
2.1.2. Polytetrafluoroethylene
2.1.3. Polyurethane
2.2. Causes of Failure of the First-Generation Polymeric Valves
2.2.1. Mechanical Degeneration
2.2.2. Polymer Degradation under Physiological Conditions
2.2.3. Thrombotic Complications
2.2.4. Calcification
2.2.5. Pannus Formation
2.2.6. New-Generation Polymer Materials
3. Novel Polymers: Material Synthesis and Properties
- (1)
- Polyhedral oligomeric silsesquioxane poly(carbonate–urea) urethane (POSS-PCU);
- (2)
- Nanocomposite graphene–PCU polymer (FGO-PCU, Hastalex);
- (3)
- Siloxane poly(urethane–urea) (SiPUU, LifePolymer);
- (4)
- Poly(styrene-b-isobutylene-b-styrene) (SIBS) and poly(styrene-b-4-vinylbenzocyclobutene-b-isobutylene-b-styrene-b-4-vinylbenzocylcobutene) (xSIBS);
- (5)
- Nanocomposite polyvinyl alcohol (PVA) and bacterial cellulose (PVA-BC);
- (6)
- Linear-low-density polyethylene (LLDPE) and hyaluronan-enhanced linear-low-density polyethylene (HA-LLDPE).
3.1. POSS-PCU
3.2. SiPUU (LifePolymer)
3.3. FGO-PCU (Hastalex)
3.4. SIBS and xSIBS
3.5. PVA and PVA-BC
3.6. LLDPE and HA-LLDPE
3.7. Trends in the Development of PHV Materials
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffey, S.; Roberts-Thomson, R.; Brown, A.; Carapetis, J.; Chen, M.; Enriquez-Sarano, M.; Zühlke, L.; Prendergast, B.D. Global epidemiology of valvular heart disease. Nat. Rev. Cardiol. 2021, 18, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, C. Heart valve disease in elderly. World J. Cardiol. 2019, 26, 71–83. [Google Scholar] [CrossRef]
- Davidson, L.J.; Davidson, C.J. Transcatheter Treatment of Valvular Heart Disease: A Review. JAMA 2021, 325, 2480–2494. [Google Scholar] [CrossRef]
- Azari, S.; Rezapour, A.; Omidi, N.; Alipour, V.; Tajdini, M.; Sadeghian, S.; Bragazzi, N.L. A systematic review of the cost-effectiveness of heart valve replacement with a mechanical versus biological prosthesis in patients with heart valvular disease. Heart Fail. Rev. 2020, 25, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Dijkman, P.E.; Emmert, M.Y.; Hoerstrup, S.P. The future of heart valve replacement: Recent developments and translational challenges for heart valve tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e323–e335. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; de Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Klyshnikov, K.Y.; Ovcharenko, E.A.; Glushkova, T.V.; Kudryavtseva, Y.A.; Barbarash, L.S. Method for non-invasive assessment of the structure of a heart valve bioprosthesis. Sib. Sci. Med. J. 2022, 42, 87–95. [Google Scholar] [CrossRef]
- Onorati, F.; Biancari, F.; de Feo, M.; Mariscalco, G.; Messina, A.; Santarpino, G.; Santini, F.; Beghi, C.; Nappi, G.; Troise, G.; et al. Mid-term results of aortic valve surgery in redo scenarios in the current practice: Results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative†. Eur. J. Cardiothorac. Surg. 2015, 47, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Poulis, N.; Zaytseva, P.; Gähwiler, E.K.N.; Motta, S.E.; Fioretta, E.S.; Cesarovic, N.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Tissue engineered heart valves for transcatheter aortic valve implantation: Current state, challenges, and future developments. Expert Rev. Cardiovasc. Ther. 2020, 18, 681–696. [Google Scholar] [CrossRef]
- Prendergast, B.D.; Redwood, S.R. Transcatheter Aortic Valve Replacement. Circulation 2019, 139, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.L.; Puehler, T.; Misso, K.; Lang, S.H.; Forbes, C.; Kleijnen, J.; Danner, M.; Kuhn, C.; Haneya, A.; Seoudy, H.; et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis: A systematic review and meta-analysis. BMJ Open 2021, 11, e054222. [Google Scholar] [CrossRef]
- Convelbo, C.; el Hafci, H.; Petite, H.; Zegdi, R. Traumatic leaflet injury: Comparison of porcine leaflet self-expandable and bovine leaflet balloon-expandable prostheses. Eur. J. Cardiothorac. Surg. 2018, 53, 1062–1067. [Google Scholar] [CrossRef]
- Steblovnik, K.; Bunc, M. Technical Aspects and Development of Transcatheter Aortic Valve Implantation. J. Cardiovasc. Dev. Dis. 2022, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Oveissi, F.; Naficy, S.; Lee, A.; Winlaw, D.S.; Dehghani, F. Materials and manufacturing perspectives in engineering heart valves: A review. Mater. Today Bio 2020, 5, 100038. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef]
- de Kort, B.J.; Koch, S.E.; Wissing, T.B.; Krebber, M.M.; Bouten, C.V.C.; Smits, A.I.P.M. Immuno-regenerative biomaterials for in situ cardiovascular tissue engineering—Do patient characteristics warrant precision engineering? Adv. Drug Deliv. Rev. 2021, 178, 113960. [Google Scholar] [CrossRef]
- Li, R.L.; Russ, J.; Paschalides, C.; Ferrari, G.; Waisman, H.; Kysar, J.W.; Kalfa, D. Mechanical considerations for polymeric heart valve development: Biomechanics, materials, design and manufacturing. Biomaterials 2019, 225, 119493. [Google Scholar] [CrossRef]
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773. [Google Scholar] [CrossRef] [Green Version]
- Rezvova, M.A.; Yuzhalin, A.E.; Glushkova, T.V.; Makarevich, M.I.; Nikishau, P.A.; Kostjuk, S.V.; Klyshnikov, K.Y.; Matveeva, V.G.; Khanova, M.Y.; Ovcharenko, E.A. Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application. Polymers 2020, 12, 2158. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Nikishau, P.A.; Makarevich, M.I.; Glushkova, T.V.; Klyshnikov, K.Y.; Akentieva, T.N.; Efimova, O.S.; Nikitin, A.P.; Malysheva, V.Y.; Matveeva, V.G.; et al. Biomaterials Based on Carbon Nanotube Nanocomposites of Poly(styrene-b-isobutylene-b-styrene): The Effect of Nanotube Content on the Mechanical Properties, Biocompatibility and Hemocompatibility. Nanomaterials 2022, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Sakudo, A. Introduction to Current Progress in Advanced Research on Prions. Curr. Issues Mol. Biol. 2020, 36, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.T.; Khair, N.; Yeats, B.; Gooden, S.; James, S.P.; Dasi, L.P. Transcatheter heart valves: A biomaterials perspective. Adv. Healthc. Mater. 2021, 10, 2100115. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Answini, G.A.; Yakubov, S.J.; Rai, B.; Smith, J.M.; Duff, S.; Shannon, F.L.; Sakwa, M.; Beith, J.; Heimansohn, D. Preliminary evaluation of a novel polymeric valve following surgical implantation for symptomatic aortic valve disease. J. Am. Coll. Cardiol. Intv. 2021, 14, 2754–2756. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A new nanocomposite copolymer based on functionalised graphene oxide for development of heart valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roe, B.B.; Moore, D. Design and fabrication of prosthetic valves. Exp. Med. Surg. 1958, 16, 177–182. [Google Scholar]
- Roe, B.B.; Owsley, J.W.; Boudoures, P.C. Experimental results with a prosthetic aortic valve. J. Thorac. Surg. 1958, 36, 563–570. [Google Scholar] [CrossRef]
- González Calderón, J.A.; Contreras López, D.; Pérez, E.; Vallejo Montesinos, J. Polysiloxanes as polymer matrices in biomedical engineering: Their interesting properties as the reason for the use in medical sciences. Polym. Bull. 2020, 77, 2749–2817. [Google Scholar] [CrossRef]
- Zare, M.; Ghomi, E.R.; Venkatraman, P.D.; Ramakrishna, S. Silicone-based biomaterials for biomedical applications: Antimicrobial strategies and 3D printing technologies. J. Appl. Polym. Sci. 2021, 138, 50969. [Google Scholar] [CrossRef]
- Roe, B.B.; Kelly, P.B.; Myers, J.L.; Moore, D.W. Tricuspid leaflet aortic valve prosthesis. Circulation. 1966, 33 (Suppl. S4), I124–I130. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.C.; Roberts, W.C.; Golden, A.; Hufnagel, C.A. Cardiac pathology after aortic valve replacement using Hufnagel trileaflet prostheses: A study of 20 necropsy patients. Am. Heart J. 1975, 89, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.B. Late follow-up studies on flexible leaflet prosthetic valves. J. Thorac. Cardiovasc. Surg. 1969, 58, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.; Correia, D.; Ribeiro, C.; Fernandes, M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE-based biomedical devices: An overview of surgical efficiency. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 302–320. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, N.S.; Morrow, A.G. A late evaluation of flexible Teflon prostheses utilized for total aortic valve replacement. Postoperative clinical, hemodynamic, and pathological assessment. J. Thorac. Cardiovasc. Surg. 1965, 49, 485–496. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Properties, Characteristics, and Applications of Expanded PTFE (ePTFE) Products. In Expanded PTFE Applications Handbook, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 163–170. [Google Scholar]
- Nistal, F.; García-Martínez, V.; Arbe, E.; Fernández, D.; Artiñano, E.; Mazorra, F.; Gallo, I. In vivo experimental assessment of polytetrafluoroethylene trileaflet heart valve prosthesis. J. Thorac. Cardiovasc. Surg. 1990, 99, 1074–1081. [Google Scholar] [CrossRef]
- Zhu, G.; Ismail, M.B.; Nakao, M.; Yuan, Q.; Yeo, J.H. Numerical and in-vitro experimental assessment of the performance of a novel designed expanded-polytetrafluoroethylene stentless bi-leaflet valve for aortic valve replacement. PLoS ONE 2019, 14, e0210780. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Yamagishi, M.; Maeda, Y.; Taniguchi, S.; Fujita, S.; Hongu, H.; Yaku, H. Long-term outcomes of expanded polytetrafluoroethylene conduits with bulging sinuses and a fan-shaped valve in right ventricular outflow tract reconstruction. J. Thorac. Cardiovasc. Surg. 2018, 155, 2567–2576. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Sung, S.C.; Kim, H.; Lee, H.D.; Kim, G.; Ko, H. Late results of right ventricular outflow tract reconstruction with a bicuspid expanded polytetrafluoroethylene valved conduit. J. Card. Surg. 2018, 33, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Quintessenza, J.A.; Jacobs, J.P.; Chai, P.J.; Morell, V.O.; Lindberg, H. Polytetrafluoroethylene Bicuspid Pulmonary Valve Implantation: Experience With 126 Patients. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamagishi, M.; Miyazaki, T.; Asada, S.; Maeda, Y.; Yaku, H.; Kado, H. Modification of expanded polytetrafluoroethylene valved conduit using the thin-type leaflets. J. Thorac. Cardiovasc. Surg. 2018, 156, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, X.; Xu, T.; Zhang, Z.; Li, X.; Han, L.; Xu, Z. Transcatheter Pulmonary Valve Replacement by Hybrid Approach Using a Novel Polymeric Prosthetic Heart Valve: Proof of Concept in Sheep. PLoS ONE 2014, 9, e100065. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-I.; Hsu, K.-H.; Li, S.-J.; Chuang, M.-K.; Luo, C.-W.; Chen, Y.-J.; Chang, C.-I. Evolution of pulmonary valve reconstruction with focused review of expanded polytetrafluoroethylene handmade valves. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, N.S.; Cooper, T.; Morrow, A.G. Complete replacement of the mitral valve. Successful clinical application of a flexible polyurethane prosthesis. J. Thorac. Cardiovasc. Surg. 1960, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, N.S. It will work: The first successful mitral valve replacement. Ann. Thorac. Surg. 1989, 48, S1–S3. [Google Scholar] [CrossRef]
- Bernacca, G.M.; Mackay, T.G.; Wilkinson, R.; Wheatley, D.J. Polyurethane heart valves: Fatigue failure, calcification, and polyurethane structure. J. Biomed. Mater. Res. 1997, 34, 371–379. [Google Scholar] [CrossRef]
- Bernacca, G.M.; Mackay, T.G.; Wheatley, D.J. In vitro function and durability of a polyurethane heart valve: Material considerations. J. Heart Valve. Dis. 1996, 5, 538–542. [Google Scholar]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Lo, H.B.; Herold, M.; Reul, H.; Mückter, H.; Taguchi, K.; Surmann, M.; Hildinger, K.H.; Lambertz, H.; de Haan, H.; Handt, S. A tricuspid polyurethane heart valve as an alternative to mechanical prostheses or bioprostheses. ASAIO Trans. 1988, 34, 839–844. [Google Scholar] [PubMed]
- Daebritz, S.H.; Sachweh, J.S.; Hermanns, B.; Fausten, B.; Franke, A.; Groetzner, J.; Klosterhalfen, B.; Messmer, B.J. Introduction of a flexible polymeric heart valve prosthesis with special design for mitral position. Circulation 2003, 108, S134–S139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Willeke, S.; Reiners, B.; Harbott, P.; Reul, H.; Rau, G. New J-3 flexible-leaflet polyurethane heart valve prosthesis with improved hydrodynamic performance. Int. J. Artif. Organs. 1991, 14, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Reul, H. A synthetic three-leaflet valve. J. Med. Eng. Technol. 1992, 16, 27–33. [Google Scholar] [CrossRef]
- Leat, M.E.; Fisher, J. A synthetic leaflet heart valve with improved opening characteristics. Med. Eng. Phys. 1994, 16, 470–476. [Google Scholar] [CrossRef]
- Mackay, T.G.; Wheatley, D.J.; Bernacca, G.M.; Fisher, A.C.; Hindle, C.S. New polyurethane heart valve prosthesis: Design, manufacture and evaluation. Biomaterials 1996, 17, 1857–1863. [Google Scholar] [CrossRef]
- Daebritz, S.H.; Fausten, B.; Hermanns, B.; Schroeder, J.; Groetzner, J.; Autschbach, R.; Messmer, B.J.; Sachweh, J.S. Introduction of a flexible polymeric heart valve prosthesis with special design for aortic position. Eur. J. Cardiothorac. Surg. 2004, 25, 946–952. [Google Scholar] [CrossRef]
- Klyshnikov, K.Y.; Onischenko, P.S.; Ovcharenko, E.A. Study of biomechanics of the heart valve leaflet apparatus using numerical simulation method. Sovrem. Tehnol. Med. 2022, 14, 6–14. [Google Scholar] [CrossRef]
- Stradins, P.; Lacis, R.; Ozolanta, I.; Purina, B.; Ose, V.; Feldmane, L.; Kasyanov, V. Comparison of biomechanical and structural properties between human aortic and pulmonary valve. Eur. J. Cardiothorac. Surg. 2004, 26, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Aguiari, P.; Fiorese, M.; Iop, L.; Gerosa, G.; Bagno, A. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Campion, G.; Hershberger, K.; Whelan, A.; Conroy, J.; Lally, C.; Murphy, B.P. A Biomechanical and microstructural analysis of bovine and porcine pericardium for use in bioprosthetic heart valves. Struct. Heart. 2021, 5, 486–496. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Klyshnikov, K.U.; Yuzhalin, A.E.; Savrasov, G.V.; Glushkova, T.V.; Vasukov, G.U.; Nushtaev, D.V.; Kudryavtseva, Y.A.; Barbarash, L.S. Comparison of xenopericardial patches of different origin and type of fixation implemented for TAVI. Int. J. Biomed. Eng. Technol. 2017, 25, 44–59. [Google Scholar] [CrossRef]
- Ghosal, K.; Chandra, A.; Praveen, G.; Snigdha, S.; Roy, S.; Agatemor, C.; Thomas, S.; Provaznik, I. Electrospinning over Solvent Casting: Tuning of Mechanical Properties of Membranes. Sci. Rep. 2018, 8, 5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbloom, S.I.; Hsu, J.H.; Fors, B.P. Controlling the shape of the molecular weight distribution for tailored tensile and rheological properties in thermoplastics and thermoplastic elastomers. J. Polym. Sci. 2022, 60, 1291–1299. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Pearce, G.; Hepton, R.; Wong, J.; Zidane, I.F.; Ma, X. An investigation into the design of a device to treat haemorrhagic stroke. Proc. Inst. Mech. Eng. H 2020, 234, 095441191989069. [Google Scholar] [CrossRef] [PubMed]
- Klyshnikov, K.Y.; Ovcharenko, E.A.; Rezvova, M.A.; Glushkova, T.V.; Barbarash, L.S. Potential benefits for using ePTFE as a material for prosthetic heart valves. Complex Issues Cardiovasc. Dis. 2018, 7, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Kodigepalli, K.M.; Thatcher, K.; West, T.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. [Google Scholar] [CrossRef]
- Ross, C.J.; Laurence, D.W.; Echols, A.L.; Babu, A.R.; Gu, T.; Duginski, G.A.; Johns, C.H.; Mullins, B.T.; Casey, K.M.; Laurence, K.A.; et al. Effects of enzyme-based removal of collagen and elastin constituents on the biaxial mechanical responses of porcine atrioventricular heart valve anterior leaflets. Acta Biomater. 2021, 135, 425–440. [Google Scholar] [CrossRef]
- Takeoka, Y.; Liu, S.; Asai, F. Improvement of mechanical properties of elastic materials by chemical methods. Sci. Technol. Adv. Mater. 2020, 21, 817–832. [Google Scholar] [CrossRef]
- Carotenuto, G.; de Nicola, S.; Palomba, M.; Pullini, D.; Horsewell, A.; Hansen, T.W.; Nicolais, L. Mechanical properties of low-density polyethylene filled by graphite nanoplatelets. Nanotechnology 2012, 23, 485705. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Lin, P.; Liu, Q. Morphology and properties of high molecular weight polyisobutylene and thermoplastic polyurethane elastomer. J. Appl. Polym. Sci. 2021, 139, 51466. [Google Scholar] [CrossRef]
- Crago, M.; Lee, A.; Farajikhah, S.; Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Winlaw, D.S.; Naficy, S. The evolution of polyurethane heart valve replacements: How chemistry translates to the clinic. Mater. Today Commun. 2022, 33, 104916. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.; Wu, Q. Structural stability of novel composite heart valve prostheses—Fatigue and wear performance. Biomed. Pharmacother. 2021, 136, 111288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, H.; Wang, Q.J. Contact creep behavior of polydimethylsiloxane and influence of load, tip size, and crosslink density. Tribol. Lett. 2013, 49, 291–299. [Google Scholar] [CrossRef]
- Riehle, N.; Athanasopulu, K.; Kutuzova, L.; Götz, T.; Kandelbauer, A.; Tovar, G.E.M.; Lorenz, G. Influence of Hard Segment Content and Diisocyanate Structure on the Transparency and Mechanical Properties of Poly(dimethylsiloxane)-Based Urea Elastomers for Biomedical Applications. Polymers 2021, 13, 212. [Google Scholar] [CrossRef]
- Daver, F.; Kajtaz, M.; Brandt, M.; Shanks, R. Creep and recovery behaviour of polyolefin-rubber nanocomposites developed for additive manufacturing. Polymers 2016, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Sui, T.; Salvati, E.; Ying, S.; Sun, G.; Dolbnya, I.P.; Dragnevski, K.; Prisacariu, C.; Korsunsky, A.M. Strain softening of nano-scale fuzzy interfaces causes Mullins effect in thermoplastic polyurethane. Sci. Rep. 2017, 7, 916. [Google Scholar] [CrossRef] [Green Version]
- Sastri, V.R. Other Polymers: Styrenics, silicones, thermoplastic elastomers, biopolymers, and thermosets. In Plastics in Medical Devices, 2nd ed.; Sastri, V.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 217–262. [Google Scholar]
- Coury, A.J. Chemical and biochemical degradation of polymers intended to be biostable. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 919–940. [Google Scholar]
- Gunatillake, P.A.; Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B. Advancements in the development of biostable polyurethanes. Polym. Rev. 2019, 59, 391–417. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Carboué, Q.; Fadlallah, S.; Lopez, M.; Allais, F. Progress in degradation behavior of most common types of functionalized polymers: A Review. Macromol. Rapid Commun. 2022, 43, 2200254. [Google Scholar] [CrossRef]
- Mazurek-Budzyńska, M.; Behl, M.; Razzaq, M.Y.; Nochel, U.; Rokicki, G.; Lendlein, A. Hydrolytic stability of aliphatic poly(carbonate-urea-urethane)s: Influence of hydrocarbon chain length in soft segment. Polym. Degrad. Stab. 2019, 161, 283–297. [Google Scholar] [CrossRef]
- Pedraza, E.; Brady, A.-C.; Fraker, C.A.; Stabler, C.L. Synthesis of macroporous poly(dimethylsiloxane) scaffolds for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 1041–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yang, J.; Ye, H.; Ding, M.; Luo, F.; Li, J.; Li, J.; Tan, H.; Fu, Q. Simultaneous Improvement of Oxidative and Hydrolytic Resistance of Polycarbonate Urethanes Based on Polydimethylsiloxane/Poly(hexamethylene carbonate) Mixed Macrodiols. Biomacromolecules 2018, 19, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation rate of biodegradable plastics at molecular level. Polym. Degrad. Stab. 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Tsuji, H.; Hayashi, T. Hydrolytic degradation and crystallization behavior of linear 2-armed and star-shaped 4-armed poly(l-lactide)s: Effects of branching architecture and crystallinity. J. Appl. Polym. Sci. 2015, 132, 41983. [Google Scholar] [CrossRef]
- Varyan, I.; Kolesnikova, N.; Xu, H.; Tyubaeva, P.; Popov, A. Biodegradability of polyolefin-based compositions: Effect of natural rubber. Polymers 2022, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic heart valve thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Zhu, G.; Wei, Y.; Yuan, Q.; Cai, L.; Nakao, M.; Yeo, J.H. In vitro assessment of the impacts of leaflet design on the hemodynamic characteristics of ePTFE pulmonary prosthetic valves. Front. Bioeng. Biotechnol. 2020, 7, 477. [Google Scholar] [CrossRef]
- Yousefi, A.; Bark, D.L.; Dasi, L.P. Effect of arched leaflets and stent profile on the hemodynamics of tri-leaflet flexible polymeric heart valves. Ann. Biomed. Eng. 2017, 45, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Piatti, F.; Sturla, F.; Marom, G.; Sheriff, J.; Claiborne, T.E.; Slepian, M.J.; Redaelli, A.; Bluestein, D. Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid–structure interaction approach. J. Biomech. 2015, 48, 3641–3649. [Google Scholar] [CrossRef] [Green Version]
- Kheradvar, A.; Groves, E.M.; Goergen, C.J.; Alavi, S.H.; Tranquillo, R.; Simmons, C.A.; Dasi, L.P.; Grande-Allen, K.J.; Mofrad, M.R.K.; Falahatpisheh, A.; et al. Emerging trends in heart valve engineering: Part II. Novel and standard technologies for aortic valve replacement. Ann. Biomed. Eng. 2015, 43, 844–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stasiak, J.R.; Serrani, M.; Biral, E.; Taylor, J.V.; Zaman, A.G.; Jones, S.; Ness, T.; de Gaetano, F.; Costantino, M.L.; Bruno, V.D.; et al. Design, development, testing at ISO standards and in vivo feasibility study of a novel polymeric heart valve prosthesis. Biomater. Sci. 2020, 8, 4467–4480. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mozafari, M. Protein adsorption on polymers. Mater. Today Commun. 2018, 17, 527–540. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, B.K.D.; Grunlan, M.A. Protein resistant polymeric biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recek, N.; Mozetic, M.; Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Vesel, A. Adsorption of proteins and cell adhesion to plasma treated polymer substrates. Int. J. Polym. Mater. 2014, 63, 685–691. [Google Scholar] [CrossRef]
- Jung, F.; Braune, S.; Lendlein, A. Haemocompatibility testing of biomaterials using human platelets. Clin. Hemorheol. Microcirc. 2013, 53, 97–115. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Borer, J.S. Bioprosthesis Failure: Is Calcification the Only Problem? JACC 2020, 76, 1749–1750. [Google Scholar] [CrossRef]
- el Khoury, G.; Vohra, H.A. Polytetrafluoroethylene leaflet extensions for aortic valve repair. Eur. J. Cardiothorac. Surg. 2012, 41, 1258–1259. [Google Scholar] [CrossRef] [Green Version]
- Hilbert, S.L.; Ferrans, V.J.; Tornita, Y.; Eidbo, E.E.; Jones, M. Evaluation of explanted polyurethane trileaflet cardiac valve prostheses. J. Thorac. Cardiovasc. Surg. 1987, 94, 419–429. [Google Scholar] [CrossRef]
- Boloori Zadeh, P.; Corbett, S.C.; Nayeb-Hashemi, H. In-vitro calcification study of polyurethane heart valves. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 35, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Al Kayal, T.; Losi, P.; Asaro, M.; Volpi, S.; Bonani, W.; Bonini, M.; Soldani, G. Analysis of oxidative degradation and calcification behavior of a silicone polycarbonate polyurethane-polydimethylsiloxane material. J. Biomed. Mater. Res. Part A 2022, 110, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Bernacca, G.M.; Mackay, T.G.; Wilkinson, R.; Wheatley, D.J. Calcification and fatigue failure in a polyurethane heart valve. Biomaterials 1995, 16, 279–285. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Campana, P.; Gerundo, G.; Caruso, A.; Grimaldi, M.G.; Russo, V.; Attena, E.; Leosco, D.; Parisi, V. The role of inflammation and metabolic risk factors in the pathogenesis of calcific aortic valve stenosis. Aging Clin. Exp. Res. 2021, 33, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Grosman-Rimon, L.; Billia, F.; Fuks, A.; Jacobs, I.; A. McDonald, M.; Cherney, D.Z.; Rao, V. New therapy, new challenges: The effects of long-term continuous flow left ventricular assist device on inflammation. Int. J. Cardiol. 2016, 215, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Ekdahl, K.N.; Larsson, R.; Nilsson, U.R.; Nilsson, B. C3 Adsorbed to a Polymer Surface Can Form an Initiating Alternative Pathway Convertase. J. Immunol. 2002, 168, 5786–5791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amore, A.; Luketich, S.K.; Raffa, G.M.; Olia, S.; Menallo, G.; Mazzola, A.; D’Accardi, F.; Grunberg, T.; Gu, X.; Pilato, M.; et al. Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. Biomaterials 2018, 150, 25–37. [Google Scholar] [CrossRef]
- Tseng, H.; Grande-Allen, K.J. Elastic fibers in the aortic valve spongiosa: A fresh perspective on its structure and role in overall tissue function. Acta Biomater. 2011, 7, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Kidane, A.G.; Burriesci, G.; Edirisinghe, M.; Ghanbari, H.; Bonhoeffer, P.; Seifalian, A.M. A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater. 2009, 5, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Farhatnia, Y.; Goh, D.; Natasha, G.; de Mel, A.; Lim, J.; Teoh, S.-H.; Malkovskiy, A.V.; Chawla, R.; Rajadas, J.; et al. Surface modification of a polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture of endothelial progenitor cells. Biointerphases 2013, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, B.; Tzamtzis, S.; Ghanbari, H.; Burriesci, G.; Seifalian, A.M. Manufacturing and hydrodynamic assessment of a novel aortic valve made of a new nanocomposite polymer. J. Biomech. 2012, 45, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B.; Easton, C.D.; Gengenbach, T.R.; Cookson, D.; Gunatillake, P.A. Morphology and surface properties of high strength siloxane poly(urethane-urea)s developed for heart valve application. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandeniyage, L.S.; Gunatillake, P.A.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B. Development of high strength siloxane poly(urethane-urea) elastomers based on linked macrodiols for heart valve application. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, S.; Farè, S.; Montagna, S.; Tanzi, M.C. Innovative polymers for TMD rehabilitation devices. J. Dent. Appl. 2014, 1, 119–123. [Google Scholar]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.-M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (‘SIBS’). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Sheriff, J.; Claiborne, T.E.; Tran, P.L.; Kothadia, R.; George, S.; Kato, Y.P.; Pinchuk, L.; Slepian, M.J.; Bluestein, D. Physical characterization and platelet interactions under shear flows of a novel thermoset polyisobutylene-based co-polymer. ACS Appl. Mater. Interfaces 2015, 7, 22058–22266. [Google Scholar] [CrossRef] [Green Version]
- McKeen, L.W. Plastics used in medical devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Modjarrad, K., Ebnesajjad, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 21–53. [Google Scholar]
- Sonker, A.K.; Tiwari, N.; Nagarale, R.K.; Verma, V. Synergistic effect of cellulose nanowhiskers reinforcement and dicarboxylic acids crosslinking towards polyvinyl alcohol properties. J. Polym. Sci. A Polym. Chem. 2016, 54, 2515–2525. [Google Scholar] [CrossRef]
- Mohammadi, H.; Boughner, D.; Millon, L.E.; Wan, W.K. Design and simulation of a poly(vinyl alcohol)-bacterial cellulose nanocomposite mechanical aortic heart valve prosthesis. Proc. Inst. Mech. Eng. H 2009, 223, 697–711. [Google Scholar] [CrossRef]
- Qiao, K.; Zheng, Y.; Guo, S.; Tan, J.; Chen, X.; Li, J.; Xu, D.; Wang, J. Hydrophilic nanofiber of bacterial cellulose guided the changes in the micro-structure and mechanical properties of nf-BC/PVA composites hydrogels. Compos. Sci. Technol. 2015, 118, 47–54. [Google Scholar] [CrossRef]
- Prawel, D.A.; Dean, H.; Forleo, M.; Lewis, N.; Gangwish, J.; Popat, K.C.; Dasi, L.P.; James, S.P. Hemocompatibility and hemodynamics of novel hyaluronan-polyethylene materials for flexible heart valve leaflets. Cardiovasc. Eng. Technol. 2014, 5, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Simon-Walker, R.; Cavicchia, J.; Prawel, D.A.; Dasi, L.P.; James, S.P.; Popat, K.C. Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1964–1975. [Google Scholar] [CrossRef]
- Zhang, M.; Pare, P.; King, R.; James, S.P. A novel ultra high molecular weight polyethylene–hyaluronan microcomposite for use in total joint replacements. II. Mechanical and tribological property evaluation. J. Biomed. Mater. Res. A 2007, 82, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; Odlyha, M.; Butler, P.E.; Seifalian, A.M. The degradative resistance of polyhedral oligomeric silsesquioxane nanocore integrated polyurethanes: An in vitro study. Biomaterials 2006, 27, 1971–1979. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef]
- Ghanbari, H.; Viatge, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites: An in vitro study. Cell Biochem. Biophys. 2006, 45, 129–136. [Google Scholar] [CrossRef]
- Ghanbari, H.; Kidane, A.G.; Burriesci, G.; Ramesh, B.; Darbyshire, A.; Seifalian, A.M. The anti-calcification potential of a silsesquioxane nanocomposite polymer under in vitro conditions: Potential material for synthetic leaflet heart valve. Acta Biomater. 2010, 6, 4249–4260. [Google Scholar] [CrossRef]
- Burriesci, G.; Marincola, F.C.; Zervides, C. Design of a novel polymeric heart valve. J. Med. Eng. Technol. 2010, 34, 7–22. [Google Scholar] [CrossRef]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; Yap, J.; Seifalian, A.M.; Burriesci, G. In vitro hydrodynamic assessment of a new transcatheter heart valve concept (the TRISKELE). J. Cardiovasc. Transl. Res. 2017, 10, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, B.; Tzamtzis, S.; Sheridan, R.; Mullen, M.J.; Yap, J.; Seifalian, A.M.; Burriesci, G. A new transcatheter heart valve concept (the TRISKELE): Feasibility in an acute preclinical model. EuroIntervention 2016, 12, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B.; Gunatillake, P.A. Hard segment composition, morphology, tensile properties and biostability of linked-macrodiol based siloxane poly(urethane urea). Mater. Today Commun. 2019, 18, 110–118. [Google Scholar] [CrossRef]
- Gallagher, G.; Padsalgikar, A.; Tkatchouk, E.; Jenney, C.; Iacob, C.; Runt, J. Environmental stress cracking performance of polyether and PDMS-based polyurethanes in an in vitro oxidation model. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Dandeniyage, L.S.; Knower, W.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B.; Gunatillake, P.A. In vitro oxidative stability of high strength siloxane poly(urethane-urea) elastomers based on linked-macrodiol. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2557–2565. [Google Scholar] [CrossRef]
- Jenney, C.; Millson, P.; Grainger, D.W.; Grubbs, R.; Gunatillake, P.; McCarthy, S.J.; Runt, J.; Beith, J. Assessment of a siloxane poly(urethane-urea) elastomer designed for implantable heart valve leaflets. Adv. Nanobiomed. Res. 2021, 1, 2000032. [Google Scholar] [CrossRef]
- Yakubov, S.J.; Wittel, J.; Johnson, G. CRT-700.20 Foldax TRIA TAVI: A novel-polymer transcatheter aortic valve: Pilot chronic ovine model study. J. Am. Coll. Cardiol. Intv. 2022, 15, S59–S60. [Google Scholar] [CrossRef]
- Pinchuk, L.; Boden, M.; Bluestein, D. The use of poly(styrene-block-isobutylene-block-styrene) and analogs for long-term implant applications. In Macromolecular Engineering; Lubnin, A., Erdodi, G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 211–235. [Google Scholar]
- Gallocher, S.L.; Aguirre, A.F.; Kasyanov, V.; Pinchuk, L.; Schoephoerster, R.T. A novel polymer for potential use in a trileaflet heart valve. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 325–334. [Google Scholar] [CrossRef]
- Wang, Q.; McGoron, A.J.; Bianco, R.; Kato, Y.; Pinchuk, L.; Schoephoerster, R.T. In-vivo assessment of a novel polymer (SIBS) trileaflet heart valve. J. Heart Valve Dis. 2010, 19, 499–505. [Google Scholar]
- Claiborne, T.E.; Sheriff, J.; Kuetting, M.; Steinseifer, U.; Slepian, M.J.; Bluestein, D. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. J. Biomech. Eng. 2013, 135, 021021. [Google Scholar] [CrossRef] [Green Version]
- Stasiak, J.; Brubert, J.; Serrani, M.; Nair, S.; de Gaetano, F.; Costantino, M.L.; Moggridge, G.D. A bio-inspired microstructure induced by slow injection moulding of cylindrical block copolymers. Soft Matter. 2014, 10, 6077–6086. [Google Scholar] [CrossRef] [Green Version]
- Claiborne, T.E.; Girdhar, G.; Gallocher-Lowe, S.; Sheriff, J.; Kato, Y.P.; Pinchuk, L.; Schoephoerster, R.T.; Jesty, J.; Bluestein, D. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna aortic bioprosthetic valves. ASAIO J. 2011, 57, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, T.E.; Xenos, M.; Sheriff, J.; Chiu, W.-C.; Soares, J.; Alemu, Y.; Gupta, S.; Judex, S.; Slepian, M.J.; Bluestein, D. Toward optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO J. 2013, 59, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, T.E.; Bluestein, D.; Schoephoerster, R.T. Development and evaluation of a novel artificial catheter-deliverable prosthetic heart valve and method for in vitro testing. Int. J. Artif. Organs 2009, 32, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Kovarovic, B.; Chiu, W.-C.; Bianchi, M.; Marom, G.; Slepian, M.J.; Bluestein, D. Novel polymeric valve for transcatheter aortic valve replacement applications: In vitro hemodynamic study. Ann. Biomed. Eng. 2019, 47, 113–125. [Google Scholar] [CrossRef]
- Rotman, O.M.; Kovarovic, B.; Bianchi, M.; Slepian, M.J.; Bluestein, D. In vitro durability and stability testing of a novel polymeric transcatheter aortic valve. ASAIO J. 2020, 66, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kovarovic, B.; Helbock, R.; Baylous, K.; Rotman, O.M.; Slepian, M.J.; Bluestein, D. Visions of TAVR future: Development and optimization of a second generation novel polymeric TAVR. J. Biomech. Eng. 2022, 144, 061008. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef]
- Jiang, H.; Campbell, G.; Boughner, D.; Wan, W.-K.; Quantz, M. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med. Eng. Phys. 2004, 26, 269–277. [Google Scholar] [CrossRef]
- Arteaga-Marrero, N.; Villa, E.; González-Fernández, J.; Martín, Y.; Ruiz-Alzola, J. Polyvinyl alcohol cryogel phantoms of biological tissues for wideband operation at microwave frequencies. PLoS ONE 2019, 14, e0219997. [Google Scholar] [CrossRef] [Green Version]
- Afghan, N. Mechanical Properties of Poly(Vinyl Alcohol) Based Blends and Composites. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2016. [Google Scholar]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol-bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Vellayappan, M.V.; Balaji, A.; Subramanian, A.P.; John, A.A.; Jaganathan, S.K.; Murugesan, S.; Mohandas, H.; Supriyanto, E.; Yusof, M. Tangible nanocomposites with diverse properties for heart valve application. Sci. Technol. Adv. Mater. 2015, 16, 033504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, H.; Goode, D.; Fradet, G.; Mequanint, K. Proposed percutaneous aortic valve prosthesis made of cryogel. Proc. Inst. Mech. Eng. H 2019, 233, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, Q.; Liu, H.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion mechanism and application progress of hydrogels. Eur. Polym. J. 2022, 173, 111277. [Google Scholar] [CrossRef]
- Vazquez, L.C.; Hagel, E.; Willenberg, B.J.; Dai, W.; Casanova, F.; Batich, C.D.; Sarntinoranont, M. Polymer-coated cannulas for the reduction of backflow during intraparenchymal infusions. J. Mater. Sci. Mater. Med. 2012, 23, 2037–2046. [Google Scholar] [CrossRef]
- Salthouse, D.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023, 155, 1–18. [Google Scholar] [CrossRef]
- Douglass, M.; Garren, M.; Devine, R.; Mondal, A.; Handa, H. Bio-inspired hemocompatible surface modifications for biomedical applications. Prog. Mater. Sci. 2022, 130, 100997. [Google Scholar] [CrossRef]
- Millon, L.E.; Mohammadi, H.; Wan, W.K. Anisotropic polyvinyl alcohol hydrogel for cardiovascular applications. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 79, 305–311. [Google Scholar] [CrossRef]
- Goode, D.; Dhaliwal, R.; Mohammadi, H. Transcatheter mitral valve replacement: State of the art. Cardiovasc. Eng. Technol. 2020, 11, 229–253. [Google Scholar] [CrossRef]
- Testa, L.; Popolo Rubbio, A.; Casenghi, M.; Pero, G.; Latib, A.; Bedogni, F. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement era. J. Am. Heart Assoc. 2019, 8, e013352. [Google Scholar] [CrossRef]
- Heitkemper, M.; Dasi, L.P. Polymeric heart valves. In Principles of Heart Valve Engineering; Kheradvar, A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 343–359. [Google Scholar]
- Zhang, M.; King, R.; Hanes, M.; James, S.P. A novel ultra high molecular weight polyethylene–hyaluronan microcomposite for use in total joint replacements. I. Synthesis and physical/chemical characterization. J. Biomed. Mater. Res. A 2006, 78, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Heitkemper, M.; Hatoum, H.; Dasi, L.P. In vitro hemodynamic assessment of a novel polymeric transcatheter aortic valve. J. Mech. Behav. Biomed. Mater. 2019, 98, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Grande-Allen, K.J. Which Biological Properties of Heart Valves Are Relevant to Tissue Engineering? Front. Cardiovasc. Med. 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, K.M.; Drews, J.D.; Breuer, C.K. Tissue-Engineered Heart Valves: A Call for Mechanistic Studies. Tissue Eng. Part B Rev. 2018, 24, 240–253. [Google Scholar] [CrossRef]
- Serrani, M.; Brubert, J.; Stasiak, J.; de Gaetano, F.; Zaffora, A.; Costantino, M.L.; Moggridge, G.D. A Computational Tool for the Microstructure Optimization of a Polymeric Heart Valve Prosthesis. J. Biomech. Eng. 2016, 138, 61001. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, F.; Khorasani, M.T.; Movahedi, M. Fabrication of polyurethane—Heparinized carbon nanotubes composite for heart valves application. Mater. Chem. Phys. 2022, 280, 125819. [Google Scholar] [CrossRef]
- Samieirad, S.; Mousavi, S.M.; Saljoughi, E. Alignment of functionalized multiwalled carbon nanotubes in forward osmosis membrane support layer induced by electric and magnetic fields. Powder Technol. 2020, 364, 538–552. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Wang, C. Multifunctional Polymer Nanocomposites Reinforced by Aligned Carbon Nanomaterials. Polymers 2018, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Elkhodiry, M.; Shi, L.; Xue, Y.; Abyaneh, M.H.; Kossar, A.P.; Giuglaris, C.; Carter, S.L.; Li, R.L.; Bacha, E.; et al. A biomimetic multilayered polymeric material designed for heart valve repair and replacement. Biomaterials 2022, 288, 121756. [Google Scholar] [CrossRef]
- Masoumi, N.; Annabi, N.; Assmann, A.; Larson, B.L.; Hjortnaes, J.; Alemdar, N.; Kharaziha, M.; Manning, K.B.; Mayer, J.E.; Khademhosseini, A. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials 2014, 35, 7774–7785. [Google Scholar] [CrossRef] [Green Version]
- Coulter, F.B.; Schaffner, M.; Faber, J.A.; Rafsanjani, A.; Smith, R.; Appa, H.; Zilla, P.; Bezuidenhout, D.; Studart, A.R. Bioinspired Heart Valve Prosthesis Made by Silicone Additive Manufacturing. Matter 2019, 1, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive Manufacturing of Metallic and Ceramic Components by the Material Extrusion of Highly-Filled Polymers: A Review and Future Perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Mao, Y.; He, Q.; Zhao, X. Designing complex architectured materials with generative adversarial networks. Sci. Adv. 2020, 6, eaaz4169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-T.; Gu, G.X. Generative Deep Neural Networks for Inverse Materials Design Using Backpropagation and Active Learning. Adv. Sci. 2020, 7, 1902607. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Biomaterial | Chemical Structure | Mechanical Properties | Surface Properties |
|---|---|---|---|
| POSS-PCU [114,115,116] |  | Tensile strength of 53.6 ± 3.4 MPa Elongation at break of 704.8 ± 38.0% Young’s modulus of 25.9 ± 1.9 MPa Tear strength of 50.0 ± 1.2 MPa | Contact angle of 100.3° ± 2.7° |
| SiPUU [117,118] |  | Tensile strength of 31.0 ± 2.4 MPa Elongation at break of 646 ± 24% Young’s modulus of 18.0 ± 0.7 MPa Tear strength of 64.0 ± 2.3 N/mm | Contact angle of 113.6° ± 0.9° |
| SIBS [119,120,121] |  | Tensile strength of 3.70 ± 0.31 MPa Elongation at break of 384.70 ± 20.78 Young’s modulus of 4.08 ± 1.17 MPa | Contact angle of 72.3° ± 3.0° |
| xSIBS [122] |  | Ultimate tensile strength of almost 35 MPa | Contact angle of 82.15° ± 0.02° |
| PVA-C [123] |  | Tensile strength of 37.3 MPa Elongation at break of 165.9% | Contact angle of 40.0° ± 2.4° |
| PVA-BC [123,124,125] |  | Tensile strength of 60.9–74.5 MPa Elongation at break of 9.6–13.8% | Contact angle data are not available |
| LLDPE [126] |  | Yield strength of 7.29 ± 0.29 Bending stiffness of 26.10 ± 3.62 Elongation at break of 582 ± 23% Young’s modulus of 73.82 ± 6.83 MPa | Contact angle of 86.8° ± 4.2° |
| HA-LLDPE [126,127,128] | Yield strength of 8.23–9.74 MPa Bending stiffness of 12.93–21.72 Elongation at break of 476–787% Young’s modulus of 76.49–99.71 MPa | Contact angle of 45° | |
| FGO-PCU [26] | not available | Tensile strength of 57.1 MPa, Elongation at break of 1004.3% Young’s modulus of 11.3 MPa | Contact angles of 106.4° ± 0.1° for the shiny surface and 85.2° ± 1.1° for the opaque surface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezvova, M.A.; Klyshnikov, K.Y.; Gritskevich, A.A.; Ovcharenko, E.A. Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. Int. J. Mol. Sci. 2023, 24, 3963. https://doi.org/10.3390/ijms24043963
Rezvova MA, Klyshnikov KY, Gritskevich AA, Ovcharenko EA. Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. International Journal of Molecular Sciences. 2023; 24(4):3963. https://doi.org/10.3390/ijms24043963
Chicago/Turabian StyleRezvova, Maria A., Kirill Y. Klyshnikov, Aleksander A. Gritskevich, and Evgeny A. Ovcharenko. 2023. "Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices" International Journal of Molecular Sciences 24, no. 4: 3963. https://doi.org/10.3390/ijms24043963
APA StyleRezvova, M. A., Klyshnikov, K. Y., Gritskevich, A. A., & Ovcharenko, E. A. (2023). Polymeric Heart Valves Will Displace Mechanical and Tissue Heart Valves: A New Era for the Medical Devices. International Journal of Molecular Sciences, 24(4), 3963. https://doi.org/10.3390/ijms24043963