Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing
Abstract
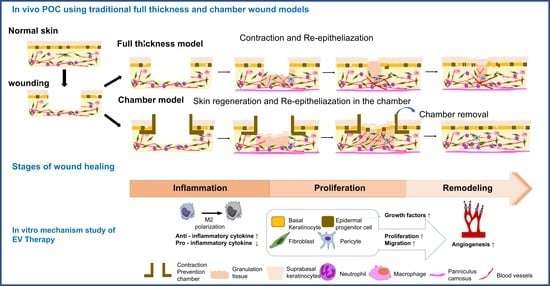
:1. Introduction
2. Results
2.1. EV Characterisation
2.2. MSC-EVs Induce Re-Epithelialization in Both Types of Wound Models
2.3. MSC-EVs Accelerate Wound Healing by Promoting the Migration of Keratinocytes
2.4. MSC-EVs Promote the Migration of Mature Fibroblasts into the Granulation Tissue
2.5. MSC-EVs Promote the Formation of New Blood Vessels in the Wound Area
2.6. In Vitro Assay for MSC-EV Effects on Four Major Cell Types, Fibroblasts, Keratocytes, Endothelial Cells, and Inflammatory Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of EV-Three-Dimensional (3D) Spheroid Cultures of WJ-MSCs
4.2. Isolation of EVs
4.3. Characterization of EVs
4.4. Two Animal Models of Cutaneous Wound
4.4.1. Conventional Full-Thickness Skin Wound Rat Model
4.4.2. Mouse Chamber Wound Model
4.5. Measurement of Wound Contraction
4.6. In Vivo Efficacy Test and Mechanism Study
4.6.1. Histological Analysis
4.6.2. Immunohistochemistry
4.6.3. Measurement of Cytokine Levels via ELISA
4.7. In Vitro Efficacy Test and Mechanism Study
4.7.1. Measurement of Nitric Oxide Production in RAW264.7 Cells
4.7.2. Fibroblast Wound Healing Assay in NIH-3T3 Cells
4.7.3. Keratinocyte Wound Healing Assay in HaCaT Cells
4.7.4. Angiogenesis Assay in Human Umbilical Vein Endothelial Cells
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
References
- Doi, H.; Kitajima, Y.; Luo, L.; Yan, C.; Tateishi, S.; Ono, Y.; Urata, Y.; Goto, S.; Mori, R.; Masuzaki, H.; et al. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci. Rep. 2016, 6, 18844. [Google Scholar] [CrossRef] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Badiavas, E.V.; Falanga, V. Treatment of chronic wounds with bone marrow-derived cells. Arch Derm. 2003, 139, 510–516. [Google Scholar] [CrossRef] [Green Version]
- Vojtassak, J.; Danisovic, L.; Kubes, M.; Bakos, D.; Jarabek, L.; Ulicna, M.; Blasko, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 2006, 27 (Suppl. S2), 134–137. [Google Scholar]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Zaher, W.; Harkness, L.; Jafari, A.; Kassem, M. An update of human mesenchymal stem cell biology and their clinical uses. Arch Toxicol. 2014, 88, 1069–1082. [Google Scholar] [CrossRef]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Lv, Q.; Deng, J.; Chen, Y.; Wang, Y.; Liu, B.; Liu, J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol. Pharm. 2020, 17, 1723–1733. [Google Scholar] [CrossRef]
- Tao, S.C.; Guo, S.C.; Li, M.; Ke, Q.F.; Guo, Y.P.; Zhang, C.Q. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl. Med. 2017, 6, 736–747. [Google Scholar] [CrossRef]
- Gao, S.; Chen, T.; Hao, Y.; Zhang, F.; Tang, X.; Wang, D.; Wei, Z.; Qi, J. Exosomal miR-135a derived from human amnion mesenchymal stem cells promotes cutaneous wound healing in rats and fibroblast migration by directly inhibiting LATS2 expression. Stem Cell Res. Ther. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.A.; Kim, S.Y.; Park, J.H.; Jo, D.G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, J.D.; Rodriguez-Menocal, L.; Guzman, W.; Candanedo, A.; Garcia-Contreras, M.; Badiavas, E.V. Bone Marrow Mesenchymal Stem Cell-Derived CD63(+) Exosomes Transport Wnt3a Exteriorly and Enhance Dermal Fibroblast Proliferation, Migration, and Angiogenesis In Vitro. Stem Cells Dev. 2017, 26, 1384–1398. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2. Stem Cell Rev. Rep. 2020, 17, 305–317. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- Johnson, J.; Shojaee, M.; Mitchell Crow, J.; Khanabdali, R. From Mesenchymal Stromal Cells to Engineered Extracellular Vesicles: A New Therapeutic Paradigm. Front. Cell Dev. Biol. 2021, 9, 705676. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Wang, X.; Wang, J.; Duan, X.; Li, R.; Peng, Y.; Ye, Q.; He, Y. Characteristics of culture-condition stimulated exosomes or their loaded hydrogels in comparison with other extracellular vesicles or MSC lysates. Front. Bioeng. Biotechnol. 2022, 16, 1016833. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 6, 6999–7010. [Google Scholar]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, M.; Araoka, T.; Hishida, T.; O’Keefe, D.D.; Takahashi, Y.; Sakamoto, A.; Sakurai, M.; Suzuki, K.; Wu, J.; Yamamoto, M.; et al. In vivo reprogramming of wound-resident cells generates skin epithelial tissue. Nature 2018, 561, 243–247. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hao, H.; Liu, J.; Fu, X.; Han, W. Repair and regeneration of skin injury by transplanting microparticles mixed with Wharton’s jelly and MSCs from the human umbilical cord. Int. J. Low Extrem. Wounds 2012, 11, 264–270. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, E.H.; Lee, H.; Sung, J.H.; Bang, O.Y. Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. Int. J. Mol. Sci. 2023, 24, 4273. https://doi.org/10.3390/ijms24054273
Kim J, Kim EH, Lee H, Sung JH, Bang OY. Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. International Journal of Molecular Sciences. 2023; 24(5):4273. https://doi.org/10.3390/ijms24054273
Chicago/Turabian StyleKim, Jieun, Eun Hee Kim, Hanbee Lee, Ji Hee Sung, and Oh Young Bang. 2023. "Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing" International Journal of Molecular Sciences 24, no. 5: 4273. https://doi.org/10.3390/ijms24054273
APA StyleKim, J., Kim, E. H., Lee, H., Sung, J. H., & Bang, O. Y. (2023). Clinical-Scale Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Wound Healing. International Journal of Molecular Sciences, 24(5), 4273. https://doi.org/10.3390/ijms24054273