1. Introduction
Protein folding is a process by which proteins reach their three-dimensional structure, their native functional form [
1]. However, some proteins do not form a defined three-dimensional structure and remain largely unfolded and disordered; these proteins are referred to as intrinsically disordered proteins (IDPs) and are often involved in cellular signaling and regulation [
2,
3]. In many cases, folding–unfolding processes are fully reversible and typically occur on a µs to ms timescale. Proteins can also misfold and form aggregates that constitute a separate phase [
2,
4]. The folding and misfolding of proteins can be investigated at equilibrium or as a function of time using various techniques including optical spectroscopy, NMR spectroscopy, and stopped-flow techniques.
A number of amyloidosis diseases, such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and type II diabetes, involve the misfolding of proteins and the formation of amyloid fibrils. These amyloid fibrils are elongated, unbranched, highly stable and ordered aggregates, which are characterized by a cross-β X-ray diffraction pattern [
2,
4,
5,
6,
7]. The fibrils are composed of assembled monomers with β-strands arranged perpendicular to the fibril axis, forming hydrogen-bonded β-sheets between monomers parallel to the fibril axis, giving rise to a strong β-sheet signal in a circular dichroism spectrum (CD) [
4,
5,
8]. Amyloid fibrils generally consist of thousands of monomers, with a diameter of up to a few nanometers (5–20 nm) and a length of several micrometers. The mature fibrils usually consist of two or more protofilaments that twist around each other [
2,
8,
9]. Structural studies of amyloid fibrils using cryo-electron microscopy (cryo-EM), atomic force microscopy (AFM), and solid-state NMR (ssNMR) have contributed detailed knowledge about the arrangement of the monomers within the fibrils and the arrangement of the β-sheets formed between different monomers along the fibril axis, as well as contacts between specific side-chains and moieties [
9].
It has been found that identical monomers can have different intra- and intermolecular interactions, giving rise to supramolecular structures with different fibril morphology (
Figure 1A). This can be affected by differences in conditions, such as temperature, pH, salt concentration, and buffer composition [
2,
9,
10]. Furthermore, it has also been shown that identical monomers can form supramolecular structures with different morphologies coexisting in the same sample; such samples are termed polymorphic [
9,
11,
12]. Different morphologies of the same peptide/protein may be related to differences in packing of the protofilaments into mature fibrils [
11,
13,
14,
15]. Differences in structures on the atomic level (packing of the monomers into protofilaments) can also cause differences in mesoscopic structure (on micrometer to nanometer scale), resulting in, for instance, different fibril length and protofilament packing, node-to-node distance, and the number of protofilaments twisting around each other [
2,
9,
10]. The morphology of the fibrils can vary, e.g., straight, twisted, or helical [
2,
9]. Additionally, different morphs will have different surface properties (
Figure 1A), which will be further addressed in this paper. This shows that despite all amyloid fibrils having in common being elongated structures with ordered cross-β-sheet motif, they can still have different local packing, different morphologies, and thus different properties even when formed from the same type of monomer. Polymorphism would, however, only exist at equilibrium if all morphs have exactly the same stability.
Free energy landscapes and protein folding funnels are often used as conceptual representations of amyloid formation [
2,
4]. The broad opening of the funnel at the top represents the many possible conformations for an unfolded protein, with high conformational entropy and high free energy. The bottom of the funnel contains fewer and narrower wells for discrete conformations, representing more stable states with lower free energies [
2,
16]. One of those wells will include the energy minimum for the native structure of the protein. However, there are also other energy minima possible, which involve the formation of partially folded states, oligomers, and misfolded aggregates, such as amorphous aggregates and amyloid fibrils [
2,
17]. The part of the folding funnel representing misfolded proteins can include many closely positioned minima of amyloid fibrils with different morphologies [
2,
12,
18]. Between the minima, there may be high or low energy barriers, which govern the kinetic stability of the different states.
Amyloids are non-covalent assemblies, and their formation is thus reversible. The relative rates of all forward and backward reactions change over time as an equilibrium is being established. For example, elongation by monomer addition is more rapid than monomer dissociation during the formation reaction in an initially supersaturated solution; however, at equilibrium, the rates are balanced so that there will be no net change in the fibril or monomer concentration. If the system is diluted, the backward reaction rate will be highest and there will be a net dissolution until a new equilibrium is established, after which the rates are again balanced. In cases of co-existing morphologies of different stability, the reversibility will ensure a net reaction flow towards the more stable morph, although this may be an overall slow process.
Above the solubility limit, the free monomer concentration at equilibrium is constant and independent of the amount of the fibrillar aggregates. Solubility is thus defined as the concentration in the fluid phase in equilibrium with a solid phase (in this case, fibrils). As a consequence of the second law of thermodynamics, the chemical potential of a substance, e.g., an amyloid peptide, will be the same in every phase of the system at equilibrium [
19,
20]. Thus, different morphs can only co-exist at equilibrium if the chemical potential of monomers in solution is identical to that in monomers in every morph. While this is unlikely in most cases, an observed polymorphism could mean that the system is not yet at equilibrium and that the kinetic barriers separating monomers and the various morphs are similar, while the barriers between morphs are high. Moreover, the apparent solubility will be lower the more stable the morph, and at infinite time, such systems should rearrange to monomorphic. Most likely, such rearrangement involves monomer dissociation from fibril ends and regrowth. In the case of in vivo-derived samples, there may be monomer heterogeneity, e.g., variation in posttranslational modification or length variants, as in the case of amyloid β peptide, meaning that there is more than one monomer type and these monomers may have different solubility and may assemble as separate morphs [
21].
α-synuclein, the focus of this study, is an intrinsically disordered protein, and its formation into amyloid fibrils is a hallmark of Parkinson’s disease [
22]. A distinct characteristic of α-synuclein is its segregated primary structure with an N-terminal amphipathic region, a central hydrophobic NAC (non-amyloid-β component) region that usually forms the fibril core, and a C-terminal acidic tail [
23,
24,
25,
26] (
Figure 1B,C). The sequence can also be divided into the three regions: the N-terminal tail, the hydrophobic fibril core, and the C-terminal tail, according to how the monomers form into fibrils, as well as the charge distribution within the sequence [
23,
24,
27,
28] (
Figure 1B). In the monomer, the two termini are slightly attracted by each other, which disfavors interactions between hydrophobic NAC regions in separate monomers [
25,
26,
29]. The 40-amino-acid-residues-long C-terminal tail protrudes from the surface of fibrils. The tails, with 15 acidic groups each, provide a “fuzzy coat” to which the N-termini from α-synuclein monomers may adsorb [
27,
30,
31]. This mode of interaction would disconnect the N- and C-termini of the individual monomer, resulting in a more extended monomer conformation and facilitating the interaction between NAC regions of monomers at the surface, potentially favoring nucleation (
Figure 1D) [
31]. The monomer fibril–interaction is reminiscent of the more thoroughly studied interaction between α-synuclein monomers and C-terminal tails decorating negatively charged phospholipid vesicles with adsorbed α-synuclein [
32,
33,
34,
35,
36,
37].
Previous studies of α-synuclein have revealed structures of different morphologies in different samples and coexisting within the same sample [
11,
14,
38]. However, in this study, we obtained fibrils of two different morphologies in different samples formed under the exact same conditions, not coexisting but originating from the same solution. The two morphs show different solubility, CD spectrum, Thioflavin-T interactions, and ultrastructure by cryo-EM. The interaction of the monomer with the different fibrillar surface was studied using solution state NMR. The data reveal two entirely different modes of interaction between monomers and fibrils depending on the fibril morphology.
Figure 1.
Structure, surface properties, sequence, and interaction modes of α-synuclein monomers and fibrils. (
A) Examples of structures of α-synuclein fibrils with two different morphologies (PDB: 6ssx and 6sst) containing two protofilaments each. Both structures are solved for residues Gly14-Gly25 and Gly36-Lys96; the N- and C-terminal tails are not part of the ordered structure. The figures are generated in Pymol. Each structure is shown from three different angles. Both structures are shown from the same angles for comparison of the surface properties of the two morphologies. Residues are colored according to their properties: acidic (red), basic (blue), hydrophobic (yellow), and non-charged polar residues (light gray) (
B) Distribution of acidic (red), basic (blue), hydrophobic (yellow), and non-charged polar residues (light gray). At the top, the sequence is divided into three regions according to how the monomer folds into the fibril structure (2NOA.pdb): the N-terminal tail (residues 1–28), the fibril core (residues 29–100), and the C-terminal tail (residues 101–140). At the bottom, the sequence is divided into the three regions according to sequence properties: the N-terminal amphipathic region (residues 1–60), the central hydrophobic NAC region (residues 61–95), and the acidic C-terminal tail (residues 96–140). (
C) The sequence of α-synuclein colored according to acidic residues (red), basic residues (blue), hydrophobic (yellow), and non-charged polar residues (black). (
D) A simplified illustration of α-synuclein monomers free in solution versus interacting with fibrils’ surface and fibril-ends based on the results in references [
25,
26,
29]. The N-terminal tail and the C-terminal tail are shown in red and blue, respectively. The fibril is shown with the acidic C-terminal tails (red) extending from the fibril core, providing an acidic “fuzzy coat” that the N-termini from α-synuclein monomers (blue) may interact with [
27,
30,
31]. The interaction between the N-termini of the monomers and the fibrils’ surface may disfavor the interaction between the N- and C-termini of the monomers, resulting in a more extended monomer conformation. This might facilitate the interaction between the NAC regions of different monomers at the fibril surface, favoring nucleation [
31]. The α-synuclein monomers can also interact with the fibril ends. Arrows represent possible interactions between N- and C-termini of the monomers, between N-termini of monomers and “fuzzy coat” of the fibrils, between a monomer and fibril end, and between central hydrophobic NAC region of different monomers that interact with the fibril surface.
Figure 1.
Structure, surface properties, sequence, and interaction modes of α-synuclein monomers and fibrils. (
A) Examples of structures of α-synuclein fibrils with two different morphologies (PDB: 6ssx and 6sst) containing two protofilaments each. Both structures are solved for residues Gly14-Gly25 and Gly36-Lys96; the N- and C-terminal tails are not part of the ordered structure. The figures are generated in Pymol. Each structure is shown from three different angles. Both structures are shown from the same angles for comparison of the surface properties of the two morphologies. Residues are colored according to their properties: acidic (red), basic (blue), hydrophobic (yellow), and non-charged polar residues (light gray) (
B) Distribution of acidic (red), basic (blue), hydrophobic (yellow), and non-charged polar residues (light gray). At the top, the sequence is divided into three regions according to how the monomer folds into the fibril structure (2NOA.pdb): the N-terminal tail (residues 1–28), the fibril core (residues 29–100), and the C-terminal tail (residues 101–140). At the bottom, the sequence is divided into the three regions according to sequence properties: the N-terminal amphipathic region (residues 1–60), the central hydrophobic NAC region (residues 61–95), and the acidic C-terminal tail (residues 96–140). (
C) The sequence of α-synuclein colored according to acidic residues (red), basic residues (blue), hydrophobic (yellow), and non-charged polar residues (black). (
D) A simplified illustration of α-synuclein monomers free in solution versus interacting with fibrils’ surface and fibril-ends based on the results in references [
25,
26,
29]. The N-terminal tail and the C-terminal tail are shown in red and blue, respectively. The fibril is shown with the acidic C-terminal tails (red) extending from the fibril core, providing an acidic “fuzzy coat” that the N-termini from α-synuclein monomers (blue) may interact with [
27,
30,
31]. The interaction between the N-termini of the monomers and the fibrils’ surface may disfavor the interaction between the N- and C-termini of the monomers, resulting in a more extended monomer conformation. This might facilitate the interaction between the NAC regions of different monomers at the fibril surface, favoring nucleation [
31]. The α-synuclein monomers can also interact with the fibril ends. Arrows represent possible interactions between N- and C-termini of the monomers, between N-termini of monomers and “fuzzy coat” of the fibrils, between a monomer and fibril end, and between central hydrophobic NAC region of different monomers that interact with the fibril surface.
![Ijms 24 05191 g001]()
2. Results
α-synuclein fibrils formed at pH 7.0 (10 mM Tris, 0.02% NaN3, 5% D2O) were found to adopt two different morphologies, here termed morphology A and morphology B. The fibril samples consisted of fibrils of either morphology A or morphology B, and there was no evidence of co-existence. This behavior was observed by four different techniques: CD, NMR, and fluorescence spectroscopy, as well as cryo-TEM, whereby the two morphologies showed differences in: CD spectra, NMR signal intensities from monomers (solubility), NMR signal intensity profiles within monomers (interaction of monomers with the fibril surface), 15N transverse relaxation rates (interaction of monomers with the fibril surface), binding to ThT, and mesoscopic structures.
2.1. Fibrils of Two Different Morphologies Examined by CD Spectroscopy, 15N−1H Signal Intensities, and Cryo-TEM
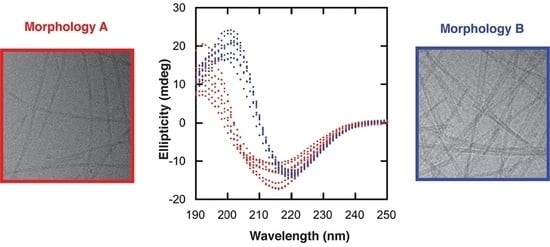
Figure 2 shows data obtained from two different fibrillar samples, one consisting of fibrils with morphology A and the other one with morphology B. It is important to emphasize that the samples presented in
Figure 2 originated from exactly the same monomeric solution. The samples were prepared as explained in
Section 4.3, and before forming the fibrils (37 °C with stirring), an equal amount of the monomeric sample was split into two separate low-binding tubes containing the same type of stir bar. After two days of incubation, both samples gave a β-sheet signal. However, they were shifted relative to each other. Morphology A gave a CD spectrum with a negative peak at 216 nm and a positive peak at 192 nm, while morphology B gave a CD spectrum that was shifted to a higher wavelength, with a negative peak at 220 nm and a positive peak at 198 nm (
Figure 2A). CD spectra were obtained for 17 independent α-synuclein fibril samples formed at the same condition. These spectra can be divided into two groups, one similar to morphology A and the other similar to morphology B (see
Figure S3).
Different behavior between the two samples was also observed by NMR spectroscopy. The
15N−
1H signal intensities of residue A140 of both samples (morphologies A and B) were compared to that of a monomeric sample in order to calculate the concentration of free monomers remaining in the two samples. Assuming that the C-terminal tail of the monomer is not affected by any transient interactions (as suggested by the transverse relaxation data, see below,
Section 2.2), the fibril sample consisting of fibrils of morphology A showed a higher solubility, with an intensity of A140 relative to the purely monomeric sample of 0.38 ± 0.04, corresponding to a monomeric concentration of 27.7 ± 3.0 µM. The sample with fibrils of morphology B showed a lower solubility, with an intensity of A140 relative to the purely monomeric sample of 0.08 ± 0.02, corresponding to a monomeric concentration of 6.0 ± 1.2 µM (
Figure 2B).
Cryo-TEM was used to evaluate ultrastructural differences between the two fibril samples (morphologies A and B) and a clear difference was seen (
Figure 2C–F). The fibrils of morphology A were thinner compared to the fibrils of morphology B, with a diameter of 11.1 ± 1.3 nm and 18.0 ± 4.9, respectively. The fibrils of morphology A seemed to be formed from two protofilaments twisting around each other while fibrils of morphology B were made from a higher number of protofilaments. Fibrils of morphology A were more twisted with a measurable node-to-node distance of 91 nm ± 8 nm. The fibrils of morphology B were straight and ribbon-like, with several protofilaments aligned next to each other; the number varied between individual fibrils, reflected in the larger standard deviation of the diameter for morphology B. The node-to-node distance was much longer and not measurable for morphology B. The sample containing fibrils of morphology B also contained a higher number of fibrils compared to the sample of fibrils of morphology A. The greater number of fibrils observed in the sample of morphology B is consistent with the lower monomer solubility as measured by NMR. Assuming that no other NMR invisible oligomers are present, there are 43 µM and 64 µM of monomers making up fibrils of morphologies A and B, respectively, in the NMR samples. As mentioned in
Section 4.7, the sample containing fibrils of morphology B had to be diluted 1:1 in the sample buffer due to a higher viscosity that caused troubles in blotting, which might be explained by the higher number of fibrils.
We tested whether the difference in concentration of monomers in equilibrium with the fibrils of the two morphologies (A and B) is the reason for the difference in the CD spectra, and if that could explain the shift of the peaks between the two morphologies. This was achieved by taking CD spectra of a fibril sample mixed with a monomeric sample at different ratios (see
Figure S4c). CD spectra of a monomeric sample and a fibril sample were also used to calculate a theoretical spectrum containing only fibrils (without soluble monomers) (see
Figure S4a,b). The results showed that the shift in the β-sheet signal between the two morphologies cannot be explained by the difference in solubility between the two morphologies.
2.2. Variation in 15N−1H Signal Intensities and Residue-Resolved 15N Transverse Relaxation Rates
Samples were prepared the same way as those analyzed in
Section 2.1. CD spectra were taken of both samples to verify the secondary structure. The two different samples gave CD spectra similar to the ones shown in
Figure 2A, where one of the samples showed a β-sheet signal corresponding to morphology A while the other sample showed a β-sheet signal shifted towards higher wavelengths, corresponding to morphology B (
Figure 3A).
15N–
1H signal intensities were measured for pure monomers before fibril formation and for monomers after formation of morphology A or B. The relative intensity of the monomer signals per amino acid residue after the formation of morphology A or B are shown in
Figure 3B. For both morphologies there is a variation in intensity over the amino acid sequence due to transient monomer–fibril interactions. In the presence of fibrils of morphology A, it is mostly the N-terminal 16 residues that are affected, and the lowest visible intensities of Met5-Leu8 are attenuated ~30-fold relative to the C-terminal A140. In the presence of fibrils of morphology B, there is a similar attenuation (~35-fold), but a very gradual increase in signal intensity from the N-terminus to around Val40, then a bit of a steeper increase until Lys102, after which the intensity remains relatively flat towards the C-terminus.
If we assume that the C-terminal tail of the monomer is not affected by any transient interactions (as suggested by the transverse relaxation data, see below), we may estimate that 30% of the monomer is free in the presence of morphology A and 10% is free in the presence of morphology B, resulting in free monomer concentrations of 21 and 7 µM, respectively. The free monomer concentration is consistent with the results shown in
Figure 2B.
15N transverse relaxation rates depend on the pico- to nanosecond mobility of the amide 15N–1H bond vectors. In short, a higher rate corresponds to a more dynamic bond vector. For 15N nuclei that experience conformational exchange between states with differing chemical shifts, the corresponding transverse relaxation rates may contain an additional exchange contribution, ΔR2. If a 15N nucleus experiences chemical shift changes of the same order of magnitude as the exchange rate, they are in the so-called intermediate exchange regime (2π ν0 Δδ ≈ kex), where ν0 is the spectrometer frequency for 15N (91.2 MHz in this case), Δδ is the 15N chemical shift difference between the two states, and kex is the exchange rate. Here, 2π ν0 Δδ is on the order of 100 s−1. These nuclei get an exchange contribution, ΔR2, to the transverse relaxation rates. If the exchange rate is on the slower side of this limit (slow on the chemical shift timescale), ΔR2 will only depend on kex, while if it is faster (fast on the chemical shift timescale), ΔR2 will depend on both kex and Δδ. For fast exchange, the measured chemical shift will be a weighted average of the shifts in the two states, while the position of the signals from the two states will be largely unaffected in slow exchange.
For slow exchange with a large object, such as a fibril, the additional exchange contribution is caused by lifetime broadening due to the unidirectional conversion of monomer to an NMR invisible fibril bound state. Here, the Δ
R2 gives an estimate of the apparent first order association rate constant,
kon, for the process. Variation in Δ
R2 along the amino acid sequence suggests a more complicated binding model [
39,
40].
15N transverse relaxation rates were measured for pure monomer before fibril formation and for monomers after the formation of morphology A or B (
Figure 3C). In the presence of fibrils of morphology A, the relaxation rates for some of the first 10 residues are higher than for the rest of the protein and much higher than for the corresponding residues in the pure monomer sample, with Δ
R2 of between 3 and 10 s
−1. Starting at residue 11 (where Δ
R2 is about 2 s
−1), the relaxation rates get gradually closer and closer to those of the pure monomer until the C-terminal Ala140 (where Δ
R2 is about 0.2 s
−1).
If we assume that the exchange contributions are due to exchange with the fibril, as has been established for acetylated α-synuclein, this means that the different parts of the monomer interact in different ways with the fibril, corroborating the conclusions made above based on the 15N−1H signal intensities.
In the presence of fibrils of morphology B, the signal intensities are so low in the N-terminal region that we can only measure relaxation rates from V66 and onwards. Here, a striking feature is that the rates in the presence of fibril are consistently lower than for the pure monomer from E104 and onwards with a peak in rates between V118 and E137. This either indicates that this part of the protein is more flexible in the presence of fibrils of morphology B or that there is some exchange contribution in the monomer that is cancelled in the presence of fibrils of this morphology. Interestingly, many of the residues from A124 and onwards also show
15N chemical shift perturbations (
Figure S5). All of this suggests that this exchange process is faster. The more flexible nature and chemical shift perturbations for the C-terminal residues may be due to the lack of interaction with the N-terminus when bound to or in the vicinity of the fibril. It is, however, not obvious exactly how this process is related to the interaction with the fibril.
2.3. Replication of Morphologies A and B and Evolution with Time
2.3.1. Replication of Morphologies A and B Using Seeds
The replication of fibrils of morphologies A and B was tested by adding 1% seeds of either morphology A or B to freshly prepared monomer solutions (
Section 4.4.2). All samples were made from the same monomeric preparation and samples were incubated at 37 °C with stirring throughout the whole experiment. After one day of incubation, samples containing seeds of morphologies A and B displayed separate β-sheet signals, i.e., the sample seeded with fibrils of morphology A had a CD spectrum corresponding to morphology A and the sample seeded with fibrils of morphology B had a CD spectrum corresponding to morphology B (
Figure 4A,B, red). This showed that the fibril morphology can be successfully replicated by seeding monomer samples.
2.3.2. Evolution of Morphologies A and B with Increased Incubation Time
CD spectra were taken as incubation went on (after 1 day, 2 days, 3 days, and 6 days) to detect any change in secondary structural content of the samples (
Figure 4 and
Figure S6). While the CD spectra of the samples containing morphology B were largely unaltered, it was found that the CD spectra of the samples initially containing morphology A became more similar to the CD spectrum of the sample containing morphology B (
Figure 4), with a negative peak around 220 nm.
Furthermore, aliquots of the samples were withdrawn at each time point, centrifuged, and the supernatant was analyzed by SDS-PAGE to compare the monomer concentration in the fibril samples to that of a pure monomeric sample (
Figure 5). After one day of incubation, the sample supplemented with seeds of morphology A had a higher concentration of monomers than the sample supplemented with fibrils of morphology B, indicating a higher solubility. This is consistent with the previous results, where morphology A showed higher solubility than morphology B when examined with NMR spectroscopy and cryo-TEM (
Figure 2 and
Figure 3).
From the SDS-PAGE (
Figure 5) and mass spectrometry with isotope standard (
Figure S7), it can also be seen that the monomer concentration in the sample seeded with fibrils of morphology A decreases with increasing incubation time. Over time, the two types of samples seem to equilibrate to the same free monomer concentration (
Figure S7). Fibrils of morphology A are thus associated with a higher apparent solubility than fibrils of morphology B. This is consistent with the time-dependent change in CD spectrum presented in
Figure 4, indicating that over time, samples seeded with fibrils of morphology A are substituted by fibrils of morphology B. This indicates that fibrils of morphology B are energetically more stable than those of morphology A (see discussion).
The bands appearing around 30 kDa correspond to dimers as centrifugation does not successfully separate monomers from dimers. The intensity of the band at 30 kDa is largely proportional to the band at 15 kDa, and thus more visible at the higher monomer concentrations in the presence of morphology A. The oligomeric distribution in the presence of morphologies A and B may be further investigated by methods, such as photo-induced cross-linking of unmodified proteins (PICUP).
2.4. Binding of Thioflavin-T to Fibrils of Morphologies A and B
The binding of ThT to amyloid fibrils restricts the rotation around the carbon–carbon bond, resulting in increased fluorescence. This makes ThT a useful probe to study amyloid fibrils [
41,
42,
43,
44]. Amyloid fibrils of different morphologies have different surface properties, affecting the flexibility around the carbon–carbon bond of the bound ThT and thus the fluorescence intensity. The interaction between ThT and the fibrils, and the resulting fluorescence intensity, has previously been shown to differ between α-synuclein fibrils of different morphologies [
45,
46]. Here, we investigated the surface properties of morphologies A and B by measuring the fluorescence emission spectra of samples containing either fibrils of morphology A or B titrated with ThT (see
Section 4.6) (
Figure 6A,B and
Figure S8). CD spectra were taken of the samples used for the experiment in order to verify the morphology present in each sample (
Figure 6C). The samples were titrated with ThT solution until the maximum intensity of the observed fluorescence spectrum started to decrease.
Figure 6A compares the ThT emission spectra with the highest fluorescence intensity. The maximum fluorescence intensity differed between the two morphologies, where the maximum ThT fluorescence intensity for the two replicates of morphology A was more than two times higher than that of the two replicates of morphology B (
Figure 6A,B). The concentration of ThT needed to reach the maximum fluorescence also differed between morphologies A and B (
Figure 6B). This study found that 35 µM and 17 µM of ThT was needed to reach the maximum fluorescence of morphologies A and B, respectively. The results indicate that the binding of ThT to the two morphologies is different, suggesting a difference in surface character between the two morphologies.
Additionally, the relative concentration of monomer in the samples was compared using SDS-PAGE to estimate the amount of monomer present in the supernatant after centrifugation of the samples. (
Figure 6D). The amount of monomer in the samples containing morphology A is higher than in the samples containing morphology B, which is consistent with results obtained by measuring the fraction of free monomers with NMR spectroscopy by calculating the relative intensity of A140 in the fibrillar samples to the intensity of a monomeric sample (
Section 2.1 and
Section 2.2). The higher ThT fluorescence of the samples containing morphology A can, therefore, not be due to a higher number of fibrils in the samples, as the samples of morphology A contain fibrils at lower concentration than the samples of morphology B.
The supernatant of the different samples after centrifugation was also analyzed by MALDI-TOF Mass Spectrometry of both intact (
Figure 6E) and trypsin-digested samples. This was performed in order to check if the monomers in supernatants of both morphologies were chemically identical after incubation and that no modifications had occurred. The data confirmed that all samples contained full-length α-synuclein without any detectable modifications.
4. Materials and Methods
4.1. Expression of α-Synuclein
4.1.1. Non-Labelled wild-Type α-Synuclein
Escherichia coli (
E. coli) BL21* pLysS Ca
2+ competent cells were used for the transformation of a pET-3a-plasmid containing the gene for wild-type α-synuclein, with
E. coli optimized codons and an ATG start codon corresponding to Met1 (purchased from GenScript, Piscataway, NJ, USA) [
27]. First, 30 to 40 µL of the competent cells were mixed with 0.7 µL of plasmid (100 ng/µL) and kept on ice for 30–60 min before it was heated for 45 s at 42 °C and placed on ice for an additional 10 min. The cells were plated and incubated overnight (ON) on LB agar plates containing chloramphenicol (30 µg/mL) and ampicillin (50 µg/mL). Negative control was performed the same way but without the plasmid. Next, the plates were stored for 8 h at 5 °C, followed by a selection of a number of small and well-isolated colonies that were each used for inoculation of a 50 mL ON culture (LB medium made from 10 g NaCl, 10 g Bacto
TM Trypton, and 10 g Bacto
TM yeast extract per L, 30 µg/mL chloramphenicol, and 50 µg/mL ampicillin) at 37 °C with shaking. The following morning, 5 mL of each ON culture was added to 500 mL of LB medium (30 µg/mL chloramphenicol and 50 µg/mL ampicillin) in 2.5 L baffled flasks and incubated at 37 °C with orbital shaking (125 rpm). When the optical density at 600 nm (OD
600 nm) reached approximately 0.9–1.0, the cultures were induced with 100 µg/mL isopropyl thio-β-D-galactoside (IPTG). The cells were harvested 4 h after the induction by centrifugation for 12 min at 6000 g and 4 °C using a JA 8.100 rotor. The pellets obtained from a total of 4 L of culture were combined, mixed with 25 mL of water, and frozen at −20 °C. Prior to harvesting, 1 mL samples were taken from each culture for analysis of the expression by SDS-PAGE (
Figure S1).
4.1.2. N-Labeled Wild-Type α-Synuclein
Expression of
15N-labeled wild-type α-synuclein was performed the same way as explained for non-labeled protein, except that M9 minimal medium was used in the day cultures. After incubation of the LB agar (with chloramphenicol (30 µg/mL) and ampicillin (50 µg/mL)) plates ON and subsequently for 8 h at 5 °C, a well-isolated single colony was transferred to 50 mL of LB medium (with 30 µg/mL chloramphenicol and 50 µg/mL ampicillin). The culture was shaken ON at 125 rpm at 37 °C. The next day, 3 mL of the ON culture was transferred to 50 mL of middle-day culture, consisting of M9 minimal medium, with
15NH
4Cl as the sole nitrogen source, supplemented with 30 µL/mL chloramphenicol and 50 µL/mL ampicillin. The OD
600 was followed, and when it had reached approximately 0.8, 50 mL of the middle-day culture was transferred to 450 mL of M9 minimal medium supplemented with 30 µL/mL chloramphenicol and 50 µL/mL at 37 °C with shaking at 120 rpm. The cultures were induced with 100 µg/mL IPTG when the OD
600 had reached approximately 0.8. The cells were harvested 4–5 h after induction by centrifugation for 12 min at 6000×
g and 4 °C in a JA 8.100 rotor. The pellets obtained from a total of 4 L of culture were combined, mixed with 25 mL of water, and frozen at −20 °C. Before harvesting, 1 mL samples were taken from each culture for testing of the expression by SDS-PAGE (see
Figure S1).
4.2. Purification of α-Synuclein
4.2.1. Handling of Cell Pellets before Chromatography
The purification was performed in the same way for the non-labeled and labeled α-synuclein. Cell pellets obtained from a total of 8 L of culture were thawed and dissolved in 100–120 mL buffer A (10 mM Tris/HCl, 10 mM EDTA, pH 7.5) and placed on ice. Next, the thawed and dissolved cell pellet was sonicated on ice using pulse sonication (1 s on, 1 s off) until the mixture became homogeneous. Thereafter, the sample was centrifuged at 15,000× g and 4 °C for 10 min (JA 25.50 rotor) and the supernatant was collected and poured into an equal volume of boiling buffer A. The temperature of the sample was measured until it reached 85 °C; afterwards, the sample container was immediately placed in ice-water slurry, stirred until it had cooled down, and then centrifuged at 15,000× g and 4 °C for 10 min (JA 25.50 rotor). This procedure precipitates and removes most of the E. coli proteins.
4.2.2. Two Steps of Ion-Exchange Chromatography
The supernatant from above was subjected to two steps of ion-exchange chromatography. The first step was performed using diethylaminoethyl (DEAE) cellulose. Buffers were always degassed, filtered (hydrophilic polypropylene membrane filters, 0.2 µm, Pall Corporation), and kept cold throughout purification; furthermore, purification was performed in a cold room. The resin was washed two times with degassed milli-Q water and then two times with buffer A, or until the pH of the resin in buffer was around 7.5. The column (3.5 cm) was packed and then equilibrated with 100 mL of buffer A. The sample was loaded slowly (approximately 2 mL/min) onto the column using a pump. Thereafter, the column was washed with a minimum of 100 mL of buffer A. At the end of the washing step, the flow rate was lowered to 1 mL/min. When the flow rate was stable (1 mL/min) the sample was eluted with a 0–0.5 M NaCl gradient in buffer A. The eluted sample was collected in fractions and analyzed with SDS-PAGE. The fractions containing most of the α-synuclein and a minimum of impurities were combined and diluted 1:1 with buffer A and subsequently purified with the second ion-exchange chromatography step, performed as described above but using 60 g of DEAE sephacel resin packed into a column with a diameter of 2.3 cm. The different fractions were first analyzed by measuring the absorbance at 280 nm, and then the fractions with absorbance at 280 nm were analyzed with SDS-PAGE to determine the purity as well as the presence of α-synuclein. The fractions containing α-synuclein and no detectable impurities were pooled and stored at −20 °C in 1 mL aliquots. The concentration of the pooled sample (in the range of 1–3 mg/mL) was measured by absorbance at 280 nm, using an extinction coefficient of ε
280 = 5800 M
−1cm
−1. The correct mass and lack of other species was confirmed using MALDI mass spectrometry (see
Figure S2).
4.3. Monomer Preparation
First, 1 mL aliquots of α-synuclein were lyophilized. To concentrate the sample, a few lyophilized aliquots were dissolved in a smaller volume of 6 M guanidinium hydrochloride, to a total volume of 1.1 mL. To make sure that the sample was fully dissolved, it was incubated for at least 1 h at RT before it was loaded (1 mL) onto a Superdex 75 Increase 10/300 GL (GE Healthcare) size exclusion column (SEC) using a fast protein liquid chromatography (FPLC) system (Bio-RAD BIOLOgic Duo Flow, Hercules, CA, USA). The sample was eluted at a flow rate of 0.7 mL/min in the experimental buffer (10 mM Tris, 0.02% NaN3, pH 7.0). The buffer was freshly prepared, filtered, and degassed before the start of each experiment. The absorbance at 280 nm was used to follow the elution of the monomers. The fractions corresponding to the center of the monomer peak (1.0–1.5 mL) were collected into low-binding tubes (Genuine Axygen Quality) and kept on ice until further use. The concentration of the monomeric sample was determined from the absorbance at 280 nm, using an extinction coefficient of ε280 = 5800 M−1cm−1. The monomeric sample was diluted to 70 µM in 10 mM Tris, 0.02% NaN3, pH 7.0. Samples analyzed with NMR spectroscopy contained 5% D2O.
4.4. Fibril Formation
4.4.1. Fibril Formation with Stirring
Fibrils were formed by incubating 70 µM of freshly purified α-synuclein monomers in a 2 mL low binding-tube (Genuine Axygen Quality) at 37 °C with continuous stirring at 700 rpm using a micro stirring bar (8 × 3 mm, polytetrafluoroethylene (PFTE)-coated, strong Alnico V magnetic core, round smooth surface (distributed by VWR International, made in UK)). The presence of fibrils was confirmed using CD spectroscopy.
4.4.2. Replication of Morphs—Seeded Fibril Formation with Stirring
For replicating the two different morphologies (termed A and B), the monomeric sample was supplemented with 1% seeds (fibrils of morphology A or B) before incubation at 37 °C and stirring at 700 rpm, as above. Seeds were tested with CD-spectroscopy and sonicated with a sonication bath for 1 min prior to use.
4.5. Far-UV Circular Dichroism (CD) Spectroscopy
Far-UV spectra were recorded using a Jasco J-815 CD spectrometer between 260 nm and 190 nm at 20 °C using a quartz cuvette with a path length of 0.1 mm, a scanning speed of 50 nm/min, continuous scanning mode, digital integration time per data point (D.I.T) of 8 s, and sensitivity set to standard. The data from three accumulations were averaged.
4.6. Binding to ThT
The binding of Thioflavin-T (ThT) to fibrils of different morphologies was investigated by titrating ThT into α-synuclein fibril samples containing fibrils of either morphology A or B. The fibril samples used for the experiment were made from 70 µM monomeric samples that had been seeded with seeds of corresponding morphology (A or B) and incubated for 1 day at 37 °C. The CD spectra of the samples were recorded before the experiment started, to verify the morphology of the samples. Samples were diluted to 10 µM (in 10 mM Tris, 0.02% NaN3, pH 7.0) and titrated with 1 mM ThT stock solution (dissolved in water). The samples were excited at 440 nm, and emission spectra were recorded from 450 to 550 nm, with excitation and emission slits set to 4 nm using a Perkin Elmer Luminescence Spectrometer LS-50B (UK). Two replicates of each morphology were titrated. The average intensity between 477 and 482 nm was plotted against the ThT concentration.
4.7. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
Two different morphologies of fibrils (morphologies A and B) formed in samples with a total monomeric concertation of 70 µM were analyzed with cryo-TEM. To ensure proper mixing, both samples were carefully pipetted up and down before they were applied to grids and frozen. The samples containing the fibrils of morphology B were diluted 1:1 in buffer prior to freezing of the sample due to higher viscosity that caused troubles in blotting. The specimens for cryo-TEM were prepared in an automatic plunge freezer system (Leica EM GP). The climate chamber temperature was kept at 21 °C, and relative humidity was ≥90% to minimize loss of solution during sample preparation. The specimens were prepared by placing 4 μL solution on glow discharged lacey formvar carbon-coated copper grids (Ted Pella) and blotted with filter paper before being plunged into liquid ethane at –183 °C. This leads to vitrified specimens, avoiding component segmentation and rearrangement and the formation of water crystals, thereby preserving original microstructures. The vitrified specimens were stored under liquid nitrogen until measured. A Fischione Model 2550 cryo transfer tomography holder was used to transfer the specimen into the electron microscope, JEM 2200FS, equipped with an in-column energy filter (Omega filter), which allows for zero-loss imaging. The acceleration voltage was 200 kV and zero-loss images were recorded digitally with a TVIPS F416 camera using SerialEM under low-dose conditions with a 10 eV energy-selecting slit in place.
The node-to-node distance and the diameter was measured using the ruler tool in the program Adobe Photoshop. The node-to-node distance was measured at 28 different positions. The diameter was measured in between the nodes, where the fibrils appeared the broadest. The diameter was measured from 100 different positions in each case.
4.8. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
The concentrations of monomers present in the fibril samples were analyzed using SDS-PAGE with NovexTM 10–20% Tricine gels (Invitrogen by Thermo Fisher Scientific). Aliquots (35 µL) were taken from the samples at different time points and centrifuged for 10 min at 14.500 rpm. The supernatant was collected (20 µL) and frozen at −20 °C for later analysis. When all samples had been collected, the frozen supernatants were thawed and 7 µL was mixed with 7 µL of loading buffer. To prepare a standard curve, 7 µL of the original monomeric sample (70, 35, and 18 µM) was also mixed with 7 µL of loading buffer. A sample volume of 10 µL was loaded onto each well, as well as 3 µL of PageRulerTM prestained protein ladder (Thermo Fisher Scientific, Waltham, MA, USA). Gels were stained using Instant BlueTM Protein Stain, Expedeon (United States Biological Life Sciences, Salem, MA; USA).
4.9. NMR Spectroscopy
All samples for NMR spectroscopy were prepared at a starting monomer concentration of 70 µM in 10 mM Tris, pH 7.0, 0.02% NaN
3, 5% D
2O (
Section 4.3). Fibrils were formed as described in
Section 4.4.1. After preparation of the individual monomeric and fibrillar samples, they were added to NMR tubes and analyzed.
In order to estimate the free monomer concentrations in the samples, the 15N−1H signal intensity of A140 was measured for pure monomer before fibril formation and for monomers in the presence of either of the two morphologies. Spectra were recorded at 298 K using an Agilent VNMRS DirectDrive spectrometer operating at a 1H frequency of 499.9 MHz and equipped with a 5 mm room temperature probe. 15N−1H signal intensities were measured using the gNhsqc pulse sequence. A total of 609 × 128 data points were collected in 48 scans for all samples.
For a more in-depth characterization of the monomer–fibril interactions, 15N−1H signal intensities and 15N transverse relaxation rates were measured for pure monomer before fibril formation and for monomers in the presence of either of the two morphologies. Spectra were recorded at 298 K using a Bruker Avance III HD 900 spectrometer (Bruker Biospin, Rheinstetten, Germany) operating at a 1H frequency of 899.8 MHz and equipped with a 5 mm cold probe. 15N−1H signal intensities were measured using the sfhmqcf3gpph pulse sequence. A total of 3072 × 256 data points were collected. 15N transverse relaxation rates were measured using a modified version of the hsqct2etf3gpsi3d pulse sequence. Spectra with relaxation delays of 0, 29, 56, 84, 112, 169, 225, 281, 337, 393, and 449 ms were recorded in an interleaved fashion. A total of 11 × 3072 × 160 data points were collected. Since the concentration of free monomer was different in the three types of samples studied, a different number of scans were collected. For pure monomer we collected 2 scans for intensities and 8 scans for relaxation rates; for morphology A we collected 2 scans for intensities and 2 times 8 scans for relaxation rates; and for morphology B we collected 256 scans for intensities and 3 times 32 scans for relaxation rates.
Spectra were processed using the topspin software (Bruker Biospin, Rheinstetten, Germany), and intensities and relaxation rates were measured in PINT [
48,
49].
4.10. Mass Spectrometry
4.10.1. MALDI Mass Spectrometry for Intact Weight Analysis
Protein samples were diluted 1:2 with 0.1% TFA before 1 µL of the protein solution was added to the MALDI target plate, mixed with 0.5 µL matrix solution (5 mg/mL a-Cyano-4-hydroxycinnamic acid in 80% Acetonitrile, 0.1% TFA) and dried in. Intact α-synuclein protein samples were analysed in linear positive mode on a MALDI mass spectrometer (Autoflex Speed, Bruker Daltonics) using external calibration with protein calibration standard I (Bruker Daltonics).
4.10.2. Digestion of Samples for Quantification Using Isotope Standard (14N vs. 15N)
Ammonium bicarbonate was added to the α-synuclein protein samples to a final concentration of 100 mM. Trypsin was then added to a tryspin:protein ratio 1:50, and the samples were digested overnight at 37 °C. Digestion was stopped by addition of formic acid (FA) to a final concentration of 0.5%.
4.10.3. LC-MSMS
Digested peptide samples were cleaned up on reversed-phase C18 micro-columns before injected to an ultra-high pressure nanoflow chromatography system (nanoElute, Bruker Daltonics). The peptides were loaded onto an Acclaim PepMap C18 (5 mm, 300 μm id, 5 μm particle diameter, 100 Å pore size) trap column (Thermo Fisher Scientific) and separated on a Bruker Pepsep Ten C18 (75 µm × 10 cm, 1.9 µm particle size) analytical column (Bruker Daltonics). Mobile phase A (2% ACN, 0.1% FA) was used with the mobile phase B (0.1% FA in ACN) for 45 min to create a gradient (from 2 to 17% B in 20 min, from 17 to 34% B in 10 min, from 34 to 95% B in 3 min, at 95% B for 12 min) at a flow rate of 400 nl/min and a column oven temperature of 50 °C. The peptides were analysed on a quadrupole time-of-flight mass spectrometer (timsTOF Pro, Bruker Daltonics) via a nano electrospray ion source (Captive Spray Source, Bruker Daltonics) in positive mode, controlled by the OtofControl 5.1 software (Bruker Daltonics). The temperature of the ion transfer capillary was 180 °C. A DDA method was used to select precursor ions for fragmentation with one TIMS-MS scan and 10 PASEF MS/MS scans. The TIMS-MS scan was acquired between 0.60–1.6 V s/cm2 and 100–1700 m/z with a ramp time of 100 ms. The 10 PASEF scans contained a maximum of 10 MS/MS scans per PASEF scan with a collision energy of 10 eV. Precursors with maximum 5 charges with intensity threshold to 5000 a. u. and a dynamic exclusion of 0.4 s were used.
4.10.4. Data Analysis
Raw data were processed using Mascot Distiller (version 2.8) and the quantification was performed using Mascot Quantitation Toolbox with the 15N Metabolic Quantitation. All data were searched against the SwissProt Database using the settings precursor ion tolerance 10 ppm, MS/MS fragment mass tolerance 0.015 Da, trypsin as protease, 1 missed cleavages site. For the quantification, six different α-synuclein peptides were used, all identified with significant individual ion scores (p < 0.005) at least two times for each light (L, 14N) and heavy (H, 15N) version of each peptide searched against the SwissProt Database. First, all six peptides are presented with an L/H value and then an average is calculated based on these to obtain a final L/H value for the protein. This final L/H value was then used to calculate the concentration of the 15N α-synuclein in the sample using the known amount of 14N α-synuclein that was added to the samples.
4.10.5. Peptides Used for Quantification Using Isotope Standard (14N vs. 15N)
The following α-synuclein peptides were used to quantify and determine the α-synuclein concentration in the samples: EGVVAAAEK (2+), EGVLYVGSK (2+), AKEGVVAAAEK (2+), TKEGVLYVGSK (2+), EGVVHGVATVAEK (2+), TKEGVVHGVATVAEK (3+).