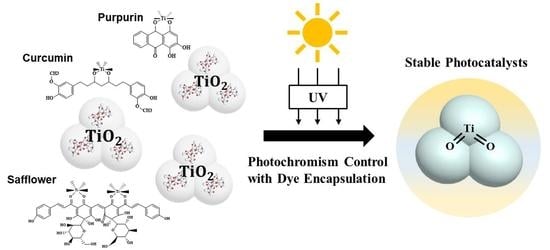
The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control
Abstract
:1. Introduction
2. Results and Discussion
2.1. Interaction between TiO2 and Natural Organic Dyes
2.2. Size Distribution after Dye Encapsulation on TiO2
2.3. Color Features of TiO2 after Dye Encapsulation
2.4. Preserving Photochromism with Dye Encapsulation
3. Materials and Methods
3.1. Materials
3.2. Self-Encapsulation of Natural Organic Dyes on TiO2
3.3. Preparation of Color Pastes
3.4. Preparation of the Red Colored Pastes
3.5. Characterization of TiO2 Hybrid Particulates
3.6. Characterization of Color Paste
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ding, H.; Zhou, H. Preparation of TiO2-Coated Barite Composite Pigments by the Hydrophobic Aggregation Method and Their Structure and Properties. Sci. Rep. 2017, 7, 10083. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, S.; Alatalo, M.; Haario, H.; Vartiainen, E.; Jalava, J.P.; Lamminmäki, R.J. Refractive Index Functions of TiO2 Nanoparticles. J. Phys. Chem. C 2013, 117, 3503–3512. [Google Scholar] [CrossRef]
- Baneshi, M.; Gonome, H.; Komiya, A.; Maruyama, S. The Effect of Particles Size Distribution on Aesthetic and Thermal Performances of Polydisperse TiO2 Pigmented Coatings: Comparison between Numerical and Experimental Results. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 594–606. [Google Scholar] [CrossRef]
- Pfaff, G.; Reynders, P. Angle-Dependent Optical Effects Deriving from Submicron Structures of Films and Pigments. Chem. Rev. 1999, 99, 1963–1981. [Google Scholar] [CrossRef]
- Farrokhpay, S. A Review of Polymeric Dispersant Stabilisation of Titania Pigment. Adv. Colloid. Interface Sci. 2009, 151, 24–32. [Google Scholar] [CrossRef]
- Costa, J.R.C.; Correia, C.; Góis, J.R.; Silva, S.M.C.; Antunes, F.E.; Moniz, J.; Serra, A.C.; Coelho, J.F.J. Efficient Dispersion of TiO2 Using Tailor Made Poly(Acrylic Acid)-Based Block Copolymers, and Its Incorporation in Water Based Paint Formulation. Prog. Org. Coat. 2017, 104, 34–42. [Google Scholar] [CrossRef]
- Bunriw, W.; Harnchana, V.; Chanthad, C.; Huynh, V.N. Natural Rubber-TiO2 Nanocomposite Film for Triboelectric Nanogenerator Application. Polymers 2021, 13, 2213. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.Y. Aging of TiO2 Nanocomposites Used in Sunscreen. Dispersion and Fate of the Degradation Products in Aqueous Environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef]
- Song, J.; Qin, J.; Qu, J.; Song, Z.; Zhang, W.; Xue, X.; Shi, Y.; Zhang, T.; Ji, W.; Zhang, R.; et al. The Effects of Particle Size Distribution on the Optical Properties of Titanium Dioxide Rutile Pigments and Their Applications in Cool Non-White Coatings. Sol. Energy Mater. Sol. Cells 2014, 130, 42–50. [Google Scholar] [CrossRef]
- Alam, A.M.; Baek, K.; Son, J.; Pei, Y.R.; Kim, D.H.; Choy, J.H.; Hyun, J.K. Generating Color from Polydisperse, Near Micron-Sized TiO2 Particles. ACS Appl. Mater. Interfaces 2017, 9, 23941–23948. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Deng, Y. Effect of Polycrystalline Structure of TiO2 Particles on the Light Scattering Efficiency. J. Colloid. Interface Sci. 2008, 319, 130–139. [Google Scholar] [CrossRef]
- Ren, R.; Wen, Z.; Cui, S.; Hou, Y.; Guo, X.; Chen, J. Controllable Synthesis and Tunable Photocatalytic Properties of Ti3+-Doped TiO2. Sci. Rep. 2015, 5, 10714. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Seo, S.; Lee, H. Recent Developments of Advanced Ti3+-Self-Doped TiO2 for Efficient Visible-Light-Driven Photocatalysis. Catalysts 2020, 10, 679. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Gong, Z.; Li, X. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons on Soil Surfaces Using TiO2 under UV Light. J. Hazard. Mater. 2008, 158, 478–484. [Google Scholar] [CrossRef]
- Smith, A.T.; Ding, H.; Gorski, A.; Zhang, M.; Gitman, P.A.; Park, C.; Hao, Z.; Jiang, Y.; Williams, B.L.; Zeng, S.; et al. Multi-Color Reversible Photochromisms via Tunable Light-Dependent Responses. Matter 2020, 2, 680–696. [Google Scholar] [CrossRef]
- Saitow, K.; Wang, Y.; Takahashi, S. Mechano-Synthesized Orange TiO2 Shows Significant Photocatalysis under Visible Light. Sci. Rep. 2018, 8, 15549. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, Characterization, Electronic Structure, and Photocatalytic Activity of Nitrogen-Doped TiO2 Nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Powell, M.J.; Palgrave, R.G.; Dunnill, C.W.; Parkin, I.P. A Fast and Effective Method for N-Doping TiO2 by Post Treatment with Liquid Ammonia: Visible Light Photocatalysis. Thin Solid. Film. 2014, 562, 223–228. [Google Scholar] [CrossRef]
- Diker, H.; Varlikli, C.; Mizrak, K.; Dana, A. Characterizations and Photocatalytic Activity Comparisons of N-Doped Nc-TiO2 Depending on Synthetic Conditions and Structural Differences of Amine Sources. Energy 2011, 36, 1243–1254. [Google Scholar] [CrossRef]
- Li, X.; Yue, P.L.; Kutal, C. Synthesis and Photocatalytic Oxidation Properties of Iron Doped Titanium Dioxide Nanosemiconductor Particles. New J. Chem. 2003, 27, 1264–1269. [Google Scholar] [CrossRef]
- Elahifard, M.R.; Ahmadvand, S.; Mirzanejad, A. Effects of Ni-Doping on the Photo-Catalytic Activity of TiO2 Anatase and Rutile: Simulation and Experiment. Mater. Sci. Semicond. Process. 2018, 84, 10–16. [Google Scholar] [CrossRef]
- He, S.; Huang, J.; Goodsell, J.L.; Angerhofer, A.; Wei, W.D. Plasmonic Nickel–TiO2 Heterostructures for Visible-Light-Driven Photochemical Reactions. Angew. Chem. 2019, 131, 6099–6102. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, J.M.; Kochuveedu, S.T.; Han, T.H.; Jeong, H.Y.; Park, M.; Yun, J.M.; Kwon, J.; No, K.; Kim, D.H.; et al. Biomineralized N-Doped CNT/TiO2 Core/Shell Nanowires for Visible Light Photocatalysis. ACS Nano 2012, 6, 935–943. [Google Scholar] [CrossRef]
- Jin, S.E.; Kim, E.J.; Kim, H.; Kim, H.; Hwang, W.; Hong, S.W. In Vitro and in Vivo Toxicological Evaluation of Transition Metal-Doped Titanium Dioxide Nanoparticles: Nickel and Platinum. Mater. Sci. Eng. C 2020, 115, 110843. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Nowacka, M.; Kołodziejczak-Radzimska, A.; Jesionowski, T. Preparation of Hybrid Pigments via Adsorption of Selected Food Dyes onto Inorganic Oxides Based on Anatase Titanium Dioxide. Dyes Pigments 2012, 94, 338–348. [Google Scholar] [CrossRef]
- Goulart, S.; Jaramillo Nieves, L.J.; Dal Bó, A.G.; Bernardin, A.M. Sensitization of TiO2 Nanoparticles with Natural Dyes Extracts for Photocatalytic Activity under Visible Light. Dyes Pigments 2020, 182, 108654. [Google Scholar] [CrossRef]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic Degradation of Organic Dyes in the Presence of Nanostructured Titanium Dioxide: Influence of the Chemical Structure of Dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Bouclé, J.; Ackermann, J. Solid-State Dye-Sensitized and Bulk Heterojunction Solar Cells Using TiO2 and ZnO Nanostructures: Recent Progress and New Concepts at the Borderline. Polym. Int. 2012, 61, 355–373. [Google Scholar] [CrossRef]
- Pastore, M.; De Angelis, F.; Cnr, I. Aggregation of Organic Dyes on TiO2. ACS Nano 2010, 4, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential; Springer: Dordrecht, The Netherlands, 2021; Volume 12, ISBN 0123456789. [Google Scholar]
- Wang, Q.; Liu, S.; Xu, L.; Du, B.; Song, L. Purification, Characterization and Bioactivities of Polysaccharides Extracted from Safflower (Carthamus tinctorius L.). Molecules 2023, 28, 596. [Google Scholar] [CrossRef]
- Lee, M.; Wakida, T.; Lee, M.S.; Pak, P.K.; Chen, J. Dyeing Transition Temperature of Wools Treated with Low Temperature Plasma, Liquid Ammonia, and High-Pressure Steam in Dyeing with Acid and Disperse Dyes. J. Appl. Polym. Sci. 2001, 80, 1058–1062. [Google Scholar] [CrossRef]
- Li, M.; Slaveykova, V.I. A Density Gradient Centrifugation Method for Rapid Separation of NanoTiO2 and TiO2 Aggregates from Microalgal Cells in Complex Mixtures with Mercury. MethodsX 2020, 7, 101057. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; David Sudol, E.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Yin, J.; Jia, J.; Yi, G. Synthesis and Photoelectric Application of AgInS2 Clusters. Mater. Lett. 2013, 111, 85–88. [Google Scholar] [CrossRef]
- Michels, L.; Richter, A.; Chellappan, R.K.; Røst, H.I.; Behsen, A.; Wells, K.H.; Leal, L.; Santana, V.; Blawid, R.; da Silva, G.J.; et al. Electronic and Structural Properties of the Natural Dyes Curcumin, Bixin and Indigo. RSC Adv. 2021, 11, 14169–14177. [Google Scholar] [CrossRef]
- Manuputty, M.Y.; Lindberg, C.S.; Dreyer, J.A.H.; Akroyd, J.; Edwards, J.; Kraft, M. Understanding the Anatase-Rutile Stability in Flame-Made TiO2. Combust. Flame 2021, 226, 347–361. [Google Scholar] [CrossRef]
- Filgueiras Rebelo de Matos, M.; Quênia Muniz Bezerra, P.; Conceição Argôlo Correia, L.; Nunes Viola, D.; de Oliveira Rios, A.; Izabel Druzian, J.; Larroza Nunes, I. Innovative Methodological Approach Using CIELab and Dye Screening for Chemometric Classification and HPLC for the Confirmation of Dyes in Cassava Flour: A Contribution to Product Quality Control. Food Chem. 2021, 365, 130446. [Google Scholar] [CrossRef] [PubMed]
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]









| Sample Name | Particle Sizes (nm) | |
|---|---|---|
| TiO2 | 94.8 ± 3.7 | |
| Purpurin | lP-TiO2 | 111.8 ± 2.6 |
| hP-TiO2 | 116.5 ± 3.4 | |
| Curcumin | lC-TiO2 | 112.1 ± 3.9 |
| hC-TiO2 | 120.2 ± 3.3 | |
| Safflower | lS-TiO2 | 88.3 ± 3.1 |
| hS-TiO2 | 113.3 ± 3.4 | |
| Sample Name | L* | a* | b* | |
|---|---|---|---|---|
| Control | TiO2 | 100 | −1.3 | 5.2 |
| Purpurin | lP-TiO2 | 78.2 | 13.5 | −1.8 |
| hP-TiO2 | 43.8 | 14.4 | −6.9 | |
| Curcumin | lC-TiO2 | 100 | −0.5 | 14.3 |
| hC-TiO2 | 77.5 | 17.9 | 31.2 | |
| Safflower | lS-TiO2 | 100 | −2.0 | 5.3 |
| hS-TiO2 | 100 | −2.4 | 10.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Shim, J.W.; Lee, J.J.; Lee, W.J. The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control. Int. J. Mol. Sci. 2023, 24, 7860. https://doi.org/10.3390/ijms24097860
Lee HJ, Shim JW, Lee JJ, Lee WJ. The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control. International Journal of Molecular Sciences. 2023; 24(9):7860. https://doi.org/10.3390/ijms24097860
Chicago/Turabian StyleLee, Hye Ju, Jong Won Shim, Jung Jin Lee, and Won Jun Lee. 2023. "The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control" International Journal of Molecular Sciences 24, no. 9: 7860. https://doi.org/10.3390/ijms24097860
APA StyleLee, H. J., Shim, J. W., Lee, J. J., & Lee, W. J. (2023). The Encapsulation of Natural Organic Dyes on TiO2 for Photochromism Control. International Journal of Molecular Sciences, 24(9), 7860. https://doi.org/10.3390/ijms24097860