Changes in Structural and Thermodynamic Properties of Starch during Potato Tuber Dormancy
Abstract
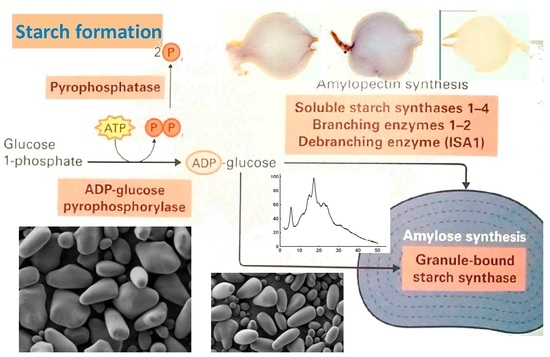
:1. Introduction
2. Results and Discussion
2.1. Starch-Related Enzymatic Activity in Dormant Tubers
2.2. Starch Granule Morphology
2.3. Starch Polymorphic Structure
2.4. Starch Thermodynamic Properties
2.5. General Considerations
3. Materials and Methods
3.1. Experimental Plants
3.2. In Situ Staining of ADP-Glucose Pyrophosphorylase (AGPase) Activity
3.3. Scanning Electron Microscopy (SEM)
3.4. X-ray Diffraction
3.5. High-Sensitivity Differential Scanning Microcalorimetry
3.6. Analysis of Starch-Related Gene Expression in Potato
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacNeill, G.J.; Mehrpouyan, S.; Minow, M.A.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution and modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef]
- Aksenova, N.P.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kolachevskaya, O.O.; Romanov, G.A. Regulation of potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2013, 60, 301–312. [Google Scholar] [CrossRef]
- Ozeretskovskaya, O.V. Cellular and molecular mechanisms of potato immunity. In Regulation of Growth and Development of Potatoes; Chailakhyan, M.K., Mokronosov, A.T., Eds.; Nauka: Moscow, Russia, 1990; pp. 131–137. (In Russian) [Google Scholar]
- Sukhova, L.S.; Korableva, N.P. Regulation of potato tubers dormancy and their disease resistance by altering hormonal balance using the ethylene donors. In Regulation of Growth and Development of Potatoes; Chailakhyan, M.K., Mokronosov, A.T., Eds.; Nauka: Moscow, Russia, 1990; pp. 138–142. (In Russian) [Google Scholar]
- Suttle, J.C. Dormancy and sprouting. In Potato Biology: Advances and Perspectives; Vreugdenhil, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 287–309. [Google Scholar]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and eco- dormancy: Physiological terminology and classification for dormancy research. Hort. Sci. 1987, 22, 371–377. [Google Scholar]
- Sheikh, F.R.; Jose-Santhi, J.; Kalia, D.; Singh, K.; Singh, R.K. Sugars as the regulators of dormancy and sprouting in geophytes. Ind. Crop. Prod. 2022, 189, 115817. [Google Scholar] [CrossRef]
- Hemberg, T. Potato rest. In Potato Physiology; Li, P.H., Ed.; Academic Press: Orlando, FL, USA, 1985; pp. 353–388. [Google Scholar]
- Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kossmann, J.; Willmitzer, L.; Romanov, G.A. Transformed potato plants as a model for studying the hormonal and carbohydrate regulation of tuberization. Russ. J. Plant Physiol. 2000, 47, 370–379. [Google Scholar]
- Shan, J.; Song, W.; Zhou, J.; Wang, X.; Xie, C.; Gao, X.; Xie, T.; Liu, J. Transcriptome analysis reveal novel genes potentially involved in photoperiodic tuberization in potato. Genomics 2013, 102, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Vreugdenhil, D.; van Lammeren, A.A.M. Cell division and cell enlargement during potato tuber formation. J. Exp. Bot. 1998, 49, 573–582. [Google Scholar] [CrossRef]
- Fernie, A.R.; Willmitzer, L. Molecular and biochemical triggers of potato tuber development. Plant Physiol. 2001, 127, 1459–1465. [Google Scholar] [CrossRef]
- Menéndez, C.M.; Ritter, E.; Schäfer-Pregl, R.; Walkemeier, B.; Kalde, A.; Salamini, F.; Gebhardt, C. Cold sweetening in diploid potato: Mapping quantitative trait loci and candidate genes. Genetics 2002, 162, 1423–1434. [Google Scholar] [CrossRef]
- Sergeeva, L.I.; Claassens, M.M.J.; Jamar, D.C.L.; van der Plas, L.H.W.; Vreugdenhil, D. Starch-related enzymes during potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2012, 59, 556–564. [Google Scholar] [CrossRef]
- Solomos, T.; Mattoo, A.K. Starch-sugar metabolism in potato (Solanum tuberosum L.) tubers in response to temperature variations. In Genetic Improvement of Solanaceous Crops. Volume I: Potato; Razdan, M.K., Mattoo, A.K., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 2005; pp. 209–234. [Google Scholar]
- Barichello, V.; Yada, R.Y.; Coffin, R.H.; Stanley, D.W. Low temperature sweetening in susceptible and resistant potatoes: Starch structure and composition. J. Food Sci. 1990, 55, 1054–1059. [Google Scholar] [CrossRef]
- Vaananen, T.; Ikonen, T.; Jokela, K.; Serimaa, R.; Pietila, L.; Pehu, E. X-ray scattering study on potato (Solanum tuberosum L.) cultivars during winter storage. Carbohydr. Polym. 2003, 54, 499–507. [Google Scholar] [CrossRef]
- Wasserman, L.A.; Sergeev, A.I.; Vasil’ev, V.G.; Plashchina, I.G.; Aksenova, N.P.; Konstantinova, T.N.; Golyanovskaya, S.A.; Sergeeva, L.I.; Romanov, G.A. Thermodynamic and structural properties of tuber starches from transgenic potato plants grown in vitro and in vivo. Carbohydr. Polym. 2015, 125, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.V.; Efremov, G.I.; Shchennikova, A.V.; Kochieva, E.Z. Dependence of the content of starch and reducing sugars on the level of expression of the genes of β-amylases StBAM1 and StBAM9 and the amylase inhibitor StAI during long-term low-temperature storage of potato tubers. Vavilov J. Genet. Breed. 2022, 26, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.R.; Kossmann, J. Starch trek: The search for yield. Front. Plant Sci. 2019, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, L.I.; Vreugdenhil, D. In situ staining of activities of enzymes involved in carbohydrate metabolism in plant tissues. J. Exp. Bot. 2002, 53, 361–370. [Google Scholar] [CrossRef]
- Appeldoorn, N.J.G.; de Bruijn, S.M.; Koot-Gronsveld, E.A.M.; Visser, R.G.F.; Vreugdenhil, D.; van der Plas, L.H.W. Developmental changes in enzymes involved in the conversion of hexose-phosphate and its subsequent metabolites during early tuberisation of potato. Plant Cell Environ. 1999, 22, 1085–1096. [Google Scholar] [CrossRef]
- Jaspreet, S.; Narpinder, S. Studies on the morphological and rheological properties of granular cold water soluble corn and potato starches. Food Hydrocoll. 2003, 17, 63–72. [Google Scholar]
- Zhou, X.; Ying, Y.; Hu, B.; Pang, Y.; Bao, J. Physicochemical properties and digestibility of endosperm starches in four indica rice mutants. Carbohydr. Polym. 2018, 195, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Han, F.; Huang, F.; Xiao, L.; Cao, S.; Liu, Z.; Thakur, K.; Han, L. Physicochemical properties and molecular structure of starches from potato cultivars of different tuber colors. Starch/Stärke 2022, 74, 11–12. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Inter. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cen, J.; Zhou, Y.; Wang, Y.; Wu, D.; Wang, Z.; Sun, J.; Shu, X. Physicochemical characterizations of five Dioscorea alata L. starches from China. Int. J. Biol. Macromol. 2023, 237, 124225. [Google Scholar] [CrossRef] [PubMed]
- Bogracheva, T.Y.; Morris, V.J.; Ring, S.G.; Hedley, C.L. The granular structure of C-type pea starch and its role in gelatinization. Biopolymers 1998, 45, 323–332. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Cairs, P.; Bogracheva, T.; Ring, S.G.; Hedley, L.L.; Morris, V.J. Determination of the polymorphic composition of smooth pea starch. Carbohydr. Polym. 1997, 31, 275–282. [Google Scholar] [CrossRef]
- Ye, F.; Xiao, L.; Liang, Y.; Zhou, Y.; Zhao, G. Spontaneous fermentation tunes the physicochemical properties of sweet potato starch by modifying the structure of starch molecules. Carbohydr. Polym. 2019, 213, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Aksenova, N.P.; Wasserman, L.A.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Krivandin, A.V.; Plashchina, I.G.; Blaszczak, W.; Fornal, J.; Romanov, G.A. Agrobacterial rol genes modify thermodynamic and structural properties of starch in microtubers of transgenic potato. Russ. J. Plant Physiol. 2010, 57, 656–663. [Google Scholar] [CrossRef]
- Donovan, J. Phase transition of the starch water system. Biopolymers 1978, 60, 263–275. [Google Scholar] [CrossRef]
- Protserov, V.A.; Karpov, V.G.; Kozhevnikov, G.O.; Wasserman, L.A.; Yuryev, V.P. Changes of thermodynamic and structural properties of potato starches (Udacha and Acrosil varieties) during biosynthesis. Starch/Stärke 2000, 52, 461–466. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Govindaraju, I.; Mazumder, N. An insight into the ultrastructural and physiochemical characterization of potato starch: A review. Am. J. Potato Res. 2020, 97, 464–476. [Google Scholar] [CrossRef]
- Chakraborty, I.; Govindaraju, I.; Kunnel, S.; Managuli, V.; Mazumder, N. Effect of storage time and temperature on digestibility, thermal, and rheological properties of retrograded rice. Gels 2023, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Vamadevan, V.; Blennow, A.; Buleon, A.; Bertoft, E. Distinct properties and structures among B-crystalline starch granules. Starch/Stärke 2018, 70, 1700240. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmed, N.; Phogat, N. Varieties, Storage Treatments and Conditions of Tubers. In Starch—Evolution and Recent Advances; Emeje, M.O., Blumenberg, M., Eds.; IntechOpen: London, UK, 2022; Chapter 5. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorov, V.M.; Kempt, T.J. Differential Scanning Calorimetry of Polymers. In Physics, Chemistry, Analysis; Ellis Horwood Ltd.: Hemel Hempstead, UK, 1994. [Google Scholar]
- Singh, N.; Inouchi, N.; Nishinari, K. Structural, thermal and viscoelastic characteristics of starches separated from normal, sugary and waxy maize. Food Hydrocoll. 2006, 20, 923–935. [Google Scholar] [CrossRef]
- Wasserman, L.A.; Papakhin, A.A.; Borodina, Z.M.; Krivandin, A.V.; Sergeev, A.I.; Tarasov, V.F. Some physico-chemical and thermodynamic characteristics of maize starches hydrolyzed by glucoamylase. Carbohydr. Polym. 2019, 212, 260–269. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Donald, A.M. The influence of amylose on starch granule structure. Int. J. Biol. Macromol. 1995, 17, 315–321. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Kozlov, S.S.; Blennow, A.; Krivandin, A.V.; Yuryev, V.P. Structural and thermodynamic properties of starches extracted from GBSS and GWD suppressed potato lines. Int. J. Biol. Macromol. 2007, 40, 449–460. [Google Scholar] [CrossRef]
- Noda, T.; Isono, N.; Krivandin, A.V.; Shatalova, O.V.; Błaszczak, W.; Yuryev, V.P. Origin of defects in assembled supramolecular structures of sweet potato starches with different amylopectin chain-length distribution. Carbohydr. Polym. 2009, 76, 400–409. [Google Scholar] [CrossRef]
- Protserov, V.A.; Wasserman, L.A.; Tester, R.F.; Debon, S.J.J.; Ezernitskaja, M.G.; Yuryev, V.P. Thermodynamic and structural properties of starches extracted from potatoes grown at different environmental temperatures. Carbohydr. Polym. 2002, 49, 271–279. [Google Scholar] [CrossRef]
- Wunderlich, B. Crystal nucleation, growth, annealing. In Macromolecular Physics; Academic Press: New York, NY, USA, 1976; Volume 2. [Google Scholar]
- Wasserman, L.A.; Eiges, N.S.; Koltysheva, G.L.; Andreev, N.R.; Karpov, V.G.; Yuryev, V.P. The application of different thermodynamic approaches for description structural features in wheat and rye starches. Starch/Stärke 2001, 55, 629–634. [Google Scholar] [CrossRef]
- Whittam, M.A.; Noel, T.R.; Ring, S.G. Melting behaviour of A- and B-type starches. Int. J. Biol. Macromol. 1990, 12, 359–362. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Cameron, R.E.; Donald, A.M. A universal feature in the structure of starch granules from different botanical sources. Starch/Stärke 1993, 45, 417–420. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; dos Santos Pereira, T.; de Andrade Freire, V.; Santiago, Â.M.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Influence of enzymatic hydrolysis on the properties of red rice starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Effect of enzymatic hydrolysis on native starch granule structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chem. 2019, 295, 138–146. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.; Wen, L.; Yang, B. Structure and physicochemical properties of native starch and resistant starch in Chinese yam (Dioscorea opposita Thunb.). Carbohydr. Polym. 2020, 237, 116188. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tohge, T.; Lalor, P.; Dockery, P.; Devaney, N.; Esteves-Ferreira, A.A.; Fernie, A.R.; Sulpice, R. Phytochrome A and B regulate primary metabolism in Arabidopsis leaves in response to light. Front. Plant Sci. 2017, 8, 1394. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, S.; Menge-Hartmann, U.; Oldenburg, E. Photosynthesis, carbohydrate metabolism, and yield of phytochrome-B-overexpressing potatoes under different light regimes. Crop Sci. 2004, 44, 131–143. [Google Scholar]
- Thiele, A.; Herold, M.; Lenk, J.; Quail, P.; Gatz, C. Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development. Plant Physiol. 1999, 120, 73–80. [Google Scholar] [CrossRef]
- Richter, M.; Augustat, S.; Schierbaum, F. Isolation, characterization and analysis of starch. In Selected Methods in Starch Chemistry; Richter, M., Augustat, S., Schierbaum, F., Eds.; Wissenschaftlliche Verlagsgesellschaft GmbH: Stuttgart, Germany, 1968; pp. 1–208. [Google Scholar]
- Krivandin, A.V.; Solov’eva, A.B.; Glagolev, N.N.; Shatalova, O.V.; Kotova, S.L. Structure alterations of perfluorinated sulfocationic membranes under the action of ethylene glycol (SAXS and WAXS studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- Andreev, N.R.; Kalistratova, E.N.; Wasserman, L.A.; Yuryev, V.P. The influence of heating rate and annealing on the melting thermodynamic parameters of some cereal starches in excess water. Starch/Stärke 1999, 50, 422–429. [Google Scholar] [CrossRef]
- Danilenko, A.N.; Shlikova, Y.V.; Yuryev, V.P. Equilibrium and co-operative unit of the melting process of native starches with different packing of the macromolecule chains in the crystallites. Biophysics 1994, 39, 427–432. [Google Scholar]
- Matveev, Y.I.; van Soest, J.J.G.; Nieman, C.; Wasserman, L.A.; Protserov, V.A.; Ezernitskaja, M.G.; Yuryev, V.P. The relationship between thermodynamic and structural properties of low and high amylose maize starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Bocharnikova, I.I.; Wasserman, L.A.; Krivandin, A.V.; Fornal, J.; Błaszczak, W.; Chernykh, V.Y.; Schiraldi, A.; Yuryev, V.P. Structure and thermodynamic melting parameters of wheat starches with different amylose content. J. Therm. Anal. Calorim. 2003, 74, 681–695. [Google Scholar] [CrossRef]
- Privalov, P.L.; Potekhin, S.A. Scanning microcalorimetry in studying temperature-induced changes in proteins. Methods Enzymol. 1986, 131, 4–51. [Google Scholar]
- Gernat, C.; Radosta, S.; Anger, H.; Damaschun, G. Crystalline parts of three different conformations detected in native and enzymatically degraded starches. Starch/Stärke 1993, 45, 309–314. [Google Scholar] [CrossRef]
- Mitsui, T.; Itoh, K.; Hori, H.; Ito, H. Biosynthesis and degradation of starch. Bullet. Facul. Agric. Niigata Univ. 2010, 62, 49–73. [Google Scholar]
- Lloyd, J.R.; Kötting, O. Starch Biosynthesis and Degradation in Plants; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Van Harsselaar, J.K.; Lorenz, J.; Senning, M.; Sonnewald, U.; Sonnewald, S. Genome-wide analysis of starch; metabolism genes in potato (Solanum tuberosum L.). BMC Genom. 2017, 18, 37. [Google Scholar] [CrossRef]









| Variant | Cultivation Conditions | Dor- mancy Time, Weeks | Tmelt, °C | Δ T | ΔHmelt, kJ/mol | ΔHvHof, kJ/mol | ν, Anhydro- Glucose Units | Lcrl, nm |
|---|---|---|---|---|---|---|---|---|
| WT | in vitro | 0 | 64.4 ± 0.0 | 7.7 | 2.9 ± 0.1 | 40.5 ± 0.1 | 14.0 ± 0.1 | 4.9 ± 0.1 |
| 4 | 64.4 ± 0.0 | 8.0 | 2.9 ± 0.1 | 39.9 ± 0.1 | 13.8 ± 0.1 | 4.8 ± 0.1 | ||
| 16 | 64.0 ± 0.0 | 8.8 | 2.5 ± 0.1 | 30.6 ± 0.1 | 12.5 ± 0.1 | 4.3 ± 0.1 | ||
| in vivo | 8 | 65.0 ± 0.1 | 7.6 | 3.5 ± 0.1 | 55.3 ± 0.2 | 15.8 ± 0.1 | 5.5 ± 0.1 | |
| 12 | 65.8 ± 0.1 | 9.1 | 3.0± 0.4 | 43.8 ± 1.1 | 15.1 ± 1.5 | 5.3 ± 0.5 | ||
| Dara 5 | in vitro | 0 | 65.1 ± 0.1 | 7.9 | 2.7 ± 0.1 | 45.5 ± 0.3 | 17.6 ± 0.3 | 6.1 ± 0.1 |
| 8 | 63.4 ±0.0 | 9.2 | 1.7 ± 0.1 | 33.8 ± 0.2 | 20.2 ± 0.1 | 7.1 ± 0.1 | ||
| in vivo | 4 | 66.0 ± 0.1 | 5.0 | 3.8 ± 0.2 | 65.1 ± 1.6 | 16.5 ± 0.2 | 5.8 ± 0.1 | |
| 8 | 65.7 ± 0.1 | 6.0 | 2.6 ± 0.1 | 52.6 ± 0.6 | 20.2 ± 0.1 | 7.0 ± 0.1 | ||
| Dara 12 | in vitro | 0 | 67.0 ± 0.1 | 7.3 | 2.8 ± 0.1 | 44.9 ± 0.3 | 16.0 ± 0.7 | 5.6 ± 0.3 |
| 7 | 66.1 ± 0.1 | 7.6 | 1.7 ± 0.1 | 38.7 ± 0.1 | 22.8 ± 0.1 | 7.9 ± 0.2 | ||
| 10 | 65.4 ± 0.1 | 8.2 | 1.7 ± 0.1 | 34.7 ± 0.1 | 20.3 ± 0.1 | 7.9 ± 0.2 |
| Variant | Cultivation Conditions | Dormancy Time, Weeks | γi × 107 J cm−2 | qi × 107 J cm−2 | si × 107 J cm−2 grad−1 |
|---|---|---|---|---|---|
| WT | in vitro in vivo | 0 | 3.233 ± 0.067 | 47.27 ± 0.67 | 0.131 ± 0.002 |
| 4 | 3.301 ± 0.067 | 48.26 ± 0.71 | 0.133 ± 0.002 | ||
| 16 | 3.020 ± 0.070 | 48.29 ± 0.36 | 0.134 ± 0.001 | ||
| 8 | 3.468 ± 0.002 | 42.80 ± 0.19 | 0.116 ± 0.005 | ||
| 12 | 3.042 ± 0.325 | 52.23 ± 12.55 | 0.145 ± 0.036 | ||
| Dara 5 | in vitro | 0 | 3.856 ± 0.053 | 66.26 ± 1.92 | 0.185 ± 0.006 |
| 8 | 5.342 ± 0.075 | 99.39 ± 1.05 | 0.280 ± 0.003 | ||
| in vivo | 4 | 3.348 ± 0.154 | 40.67 ± 7.23 | 0.110 ± 0.021 | |
| 8 | 4.066 ± 0.004 | 75.32 ± 0.93 | 0.210 ± 0.003 | ||
| Dara 12 | in vitro | 0 | 2.656 ± 0.073 | 56.65 ± 3.41 | 0.160 ± 0.099 |
| 7 | 4.357 ± 0.054 | 110.56 ± 0.07 | 0.313 ± 0.005 | ||
| 10 | 4.272 ± 0.069 | 99.36 ± 0.35 | 0.281 ± 0.000 |
| Variant | Cultivation Conditions | Dormancy Duration, Weeks | Melting Temperature (°C) and Proportion (%) | |||
|---|---|---|---|---|---|---|
| Low Temperature Transition | High Temperature Transition | |||||
| T1, °C | α1, % | T2, °C | α2, % | |||
| WT | in vitro | 0 | 58.1 | 11.5 | 64.4 | 88.5 |
| 4 | 61.3 | 14.0 | 64.7 | 86.0 | ||
| 16 | 61.7 | 19.3 | 64.1 | 80.7 | ||
| in vivo | 8 | 62.6 | 12.2 | 65.2 | 87.8 | |
| 12 | 62.3 | 15.3 | 66.1 | 84.7 | ||
| Dara 5 | in vitro | 0 | 62.5 | 12.4 | 65.3 | 87.6 |
| 8 | 63.2 | 83.4 | 66.5 | 16.6 | ||
| in vivo | 4 | 66.0 | 85.5 | 68.8 | 14.4 | |
| 8 | 65.9 | 88.0 | 68.9 | 12.0 | ||
| Dara 12 | in vitro | 0 | 61.5 | 9.1 | 66.9 | 90.9 |
| 7 | 66.1 | 87.4 | 68.9 | 12.6 | ||
| 12 | 65.8 | 90.4 | 68.7 | 9.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasserman, L.A.; Kolachevskaya, O.O.; Krivandin, A.V.; Filatova, A.G.; Gradov, O.V.; Plashchina, I.G.; Romanov, G.A. Changes in Structural and Thermodynamic Properties of Starch during Potato Tuber Dormancy. Int. J. Mol. Sci. 2023, 24, 8397. https://doi.org/10.3390/ijms24098397
Wasserman LA, Kolachevskaya OO, Krivandin AV, Filatova AG, Gradov OV, Plashchina IG, Romanov GA. Changes in Structural and Thermodynamic Properties of Starch during Potato Tuber Dormancy. International Journal of Molecular Sciences. 2023; 24(9):8397. https://doi.org/10.3390/ijms24098397
Chicago/Turabian StyleWasserman, Lyubov A., Oksana O. Kolachevskaya, Alexey V. Krivandin, Anna G. Filatova, Oleg V. Gradov, Irina G. Plashchina, and Georgy A. Romanov. 2023. "Changes in Structural and Thermodynamic Properties of Starch during Potato Tuber Dormancy" International Journal of Molecular Sciences 24, no. 9: 8397. https://doi.org/10.3390/ijms24098397
APA StyleWasserman, L. A., Kolachevskaya, O. O., Krivandin, A. V., Filatova, A. G., Gradov, O. V., Plashchina, I. G., & Romanov, G. A. (2023). Changes in Structural and Thermodynamic Properties of Starch during Potato Tuber Dormancy. International Journal of Molecular Sciences, 24(9), 8397. https://doi.org/10.3390/ijms24098397