Release of Pro-Inflammatory/Angiogenic Factors by Retinal Microvascular Cells Is Mediated by Extracellular Vesicles Derived from M1-Activated Microglia
Abstract
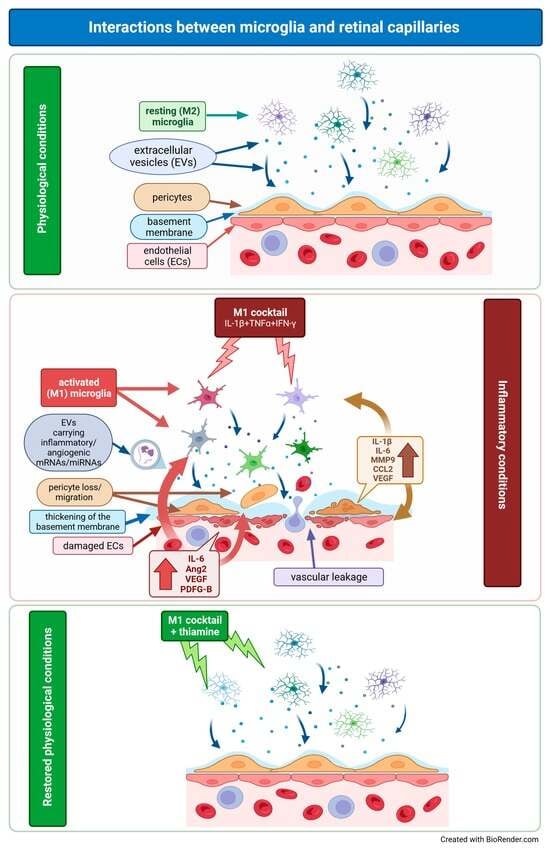
:1. Introduction
2. Results
2.1. EV Characterization
2.2. EV Expression of Pro-Inflammatory mRNAs and miRNAs
2.3. Functional Changes in Retinal Microvascular Cells Exposed to Microglia-Derived EVs
2.4. Release of Pro-Inflammatory/Pro-Angiogenic Factors by HRPs/HRECs following Exposure to Microglia-Derived EVs
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. EV Production and Collection
4.3. Protein-Content EV Characterization
4.4. mRNA Expression
4.5. miRNA Expression
4.6. Cell Function Experiments
4.7. Release of Pro-Inflammatory/Pro-Angiogenic Factors
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teo, Z.; Tham, Y.; Yu, M.; Chee, M.; Rim, T.; Cheung, N.; Bikbov, M.; Wang, Y.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Valverde, Á. Modulation of microglia in the retina: New insights into diabetic retinopathy. Acta Diabetol. 2017, 54, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Arroba, A.I.; Campos-Caro, A.; Aguilar-Diosdado, M.; Valverde, Á. IGF-1, Inflammation and Retinal Degeneration: A Close Network. Front. Aging Neurosci. 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Arroba, A.I.; Beltramo, E.; Valverde, A.M.; Porta, M. Somatostatin protects human retinal pericytes from inflammation mediated by microglia. Exp. Eye Res. 2017, 164, 46–54. [Google Scholar] [CrossRef]
- Madeira, M.; Boia, R.; Santos, P.; Ambrósio, A.; Santiago, A. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Tetta, C. Paracrine/endocrine mechanism of stem cells on kidney repair: Role of microvesicle-mediated transfer of genetic information. Curr. Opin. Nephrol. Hypertens 2010, 19, 7–12. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Mordà, D.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Human retinal secretome: A cross-link between mesenchymal and retinal cells. World J. Stem Cells 2023, 15, 665–686. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, W.; Li, B.; Zou, J.; Li, Y.; Xiao, Y.; He, Y.; Yoshida, S.; Zhou, Y. Exosomal non-coding RNAs in angiogenesis: Functions, mechanisms and potential clinical applications. Heliyon 2023, 9, e18626. [Google Scholar] [CrossRef] [PubMed]
- Diamant, M.; Nieuwland, R.; Pablo, R.F.; Sturk, A.; Smit, J.W.; Radder, J.K. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 2002, 106, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Müller, G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab. Syndr. Obes. 2012, 5, 247–282. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Gu, T.; Zhang, Z.; Wu, T.; Wang, J.; Bi, Y. The role of miRNAs carried by extracellular vesicles in type 2 diabetes and its complications. J. Diabetes 2023, 15, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hua, S.; You, L.; Zhong, T. Secretome Derived from Mesenchymal Stem/Stromal Cells: A Promising Strategy for Diabetes and its Complications. Curr. Stem Cell Res. Ther. 2023; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M.; Beltramo, E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp. Eye Res. 2019, 184, 56–63. [Google Scholar] [CrossRef]
- Aires, I.; Ribeiro-Rodrigues, T.; Boia, R.; Catarino, S.; Girão, H.; Ambrósio, A.; Santiago, A. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia 2020, 68, 2705–2724. [Google Scholar] [CrossRef]
- Hammes, H.P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D.; et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef]
- Beltramo, E.; Berrone, E.; Tarallo, S.; Porta, M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol. 2008, 45, 131–141. [Google Scholar] [CrossRef]
- Bowyer, J.F.; Tranter, K.M.; Sarkar, S.; Hanig, J.P. Microglial activation and vascular responses that are associated with early thalamic neurodegeneration resulting from thiamine deficiency. Neurotoxicology 2018, 65, 98–110. [Google Scholar] [CrossRef]
- Bozic, I.; Savic, D.; Stevanovic, I.; Pekovic, S.; Nedeljkovic, N.; Lavrnja, I. Benfotiamine upregulates antioxidative system in activated BV-2 microglia cells. Front. Cell. Neurosci. 2015, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, S.; Beltramo, E.; Berrone, E.; Dentelli, P.; Porta, M. Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetol. 2010, 47, 105–111. [Google Scholar] [CrossRef]
- Mazzeo, A.; Porta, M.; Beltramo, E. Characterization of an Immortalized Human Microglial Cell Line as a Tool for the Study of Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 5745. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.-B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Aires, I.; Ribeiro-Rodrigues, T.; Boia, R.; Ferreira-Rodrigues, M.; Girão, H.; Ambrósio, A.; Santiago, A. Microglial Extracellular Vesicles as Vehicles for Neurodegeneration Spreading. Biomolecules 2021, 11, 770. [Google Scholar] [CrossRef]
- Fabbri, M.; Croce, C.M.; Calin, G.A. MicroRNAs. Cancer J. 2008, 14, 1–6. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 513–521. [Google Scholar] [CrossRef]
- Snowhite, I.V.; Allende, G.; Sosenko, J.; Pastori, R.L.; Messinger Cayetano, S.; Pugliese, A. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 2017, 60, 1409–1422. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Toto, L.; Cipollone, F.; Santovito, D.; Carpineto, P.; Mastropasqua, L. Role of microRNAs in the modulation of diabetic retinopathy. Prog. Retin Eye Res. 2014, 43, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Ishida, S.; Inoue, M.; Obata, K.; Oguchi, Y.; Okada, Y.; Ikeda, E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Inagaki, M.; Fujita, Y.; Kimoto, T.; Tanabe-Fujimura, C.; Zou, K.; Liu, J.; Liu, S.; Komano, H. ATP increases the migration of microglia across the brain endothelial cell monolayer. Biosci. Rep. 2016, 36, e00318. [Google Scholar] [CrossRef]
- Spampinato, S.; Costantino, G.; Merlo, S.; Canonico, P.; Sortino, M. Microglia Contributes to BAF-312 Effects on Blood-Brain Barrier Stability. Biomolecules 2022, 12, 1174. [Google Scholar] [CrossRef]
- Yang, T.; Guo, L.; Fang, Y.; Liang, M.; Zheng, Y.; Pan, M.; Meng, C.; Liu, G. Pericytes of Indirect Contact Coculture Decrease Integrity of Inner Blood-Retina Barrier Model In Vitro by Upgrading MMP-2/9 Activity. Dis. Markers 2021, 2021, 7124835. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Ge, S.; Lemire, Y.; Jellison, E.; Serwanski, D.; Ruddle, N.; Pachter, J. Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflamm. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Annese, T.; Petrosino, V.; Longo, G.; Girolamo, F.; de Trizio, I.; d’Amati, A.; Uccelli, A.; Kerlero de Rosbo, N.; Virgintino, D. Microglia-derived CCL2 has a prime role in neocortex neuroinflammation. Fluids Barriers CNS 2022, 19, 68. [Google Scholar] [CrossRef]
- Medina-Flores, F.; Hurtado-Alvarado, G.; Deli, M.; Gómez-González, B. The Active Role of Pericytes During Neuroinflammation in the Adult Brain. Cell. Mol. Neurobiol. 2023, 43, 525–541. [Google Scholar] [CrossRef]
- Urbančič, M.; Petrovič, D.; Živin, A.; Korošec, P.; Fleža, M.; Petrovič, M. Correlations between vitreous cytokine levels and inflammatory cells in fibrovascular membranes of patients with proliferative diabetic retinopathy. Mol. Vis. 2020, 26, 472–482. [Google Scholar]
- Fu, J.; Zhu, J. Relationship among Serum Homocysteine, Intercellular Adhesion Molecule-1, Monocyte Chemoattractant Protein-1, and Visual Impairment in Diabetic Macular Edema. J. Coll. Physicians Surg. Pak. JCPSP 2022, 32, 57–60. [Google Scholar] [CrossRef] [PubMed]
- McCall, M.; Illei, P.; Halushka, M. Complex Sources of Variation in Tissue Expression Data: Analysis of the GTEx Lung Transcriptome. Am. J. Hum. Genet. 2016, 99, 624–635. [Google Scholar] [CrossRef] [PubMed]
- de Rie, D.; Abugessaisa, I.; Alam, T.; Arner, E.; Arner, P.; Ashoor, H.; Åström, G.; Babina, M.; Bertin, N.; Burroughs, A.; et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nat. Biotechnol. 2017, 35, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh-Gavgani, E.; Oladghaffari, M.; Bahramian, S.; Majidazar, R.; Dolati, S. MicroRNA-21: A critical underestimated molecule in diabetic retinopathy. Gene 2023, 859, 147212. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Devier, D.J.; Lovera, J.F.; Lukiw, W.J. Increase in NF-κB-sensitive miRNA-146a and miRNA-155 in multiple sclerosis (MS) and pro-inflammatory neurodegeneration. Front. Mol. Neurosci. 2015, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.L.; Guedes, J.R.; de Lima, M.C. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr. Opin. Pharmacol. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Song, S.; Xue, H.; Shi, D.; Liu, C.; Liu, H. Regulatory T cells in the pathogenesis of type 2 diabetes mellitus retinopathy by miR-155. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2010–2015. [Google Scholar]
- Thornalley, P.J.; Babaei-Jadidi, R.; Al Ali, H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef]
- Babaei-Jadidi, R.; Karachalias, N.; Ahmed, N.; Battah, S.; Thornalley, P.J. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 2003, 52, 2110–2120. [Google Scholar] [CrossRef]
- Ryoo, H.; Bergmann, A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008797. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.; Huang, Q.; Li, F.; Liu, X.; Li, C. Cell death-stimulated cell proliferation: A tissue regeneration mechanism usurped by tumors during radiotherapy. Semin. Radiat. Oncol. 2013, 23, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ping, S.; Li, Y.; Liu, S.; Zhang, Z.; Wang, J.; Zhou, Y.; Liu, K.; Huang, J.; Chen, D.; Li, C. Simultaneous Increases in Proliferation and Apoptosis of Vascular Smooth Muscle Cells Accelerate Diabetic Mouse Venous Atherosclerosis. PLoS ONE 2015, 10, e0141375. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Dohgu, S.; Takata, F.; Machida, T.; Bölükbaşi Hatip, F.; Hatip-Al-Khatib, I.; Yamauchi, A.; Kataoka, Y. TNF-α-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IκB-NFκB and JAK-STAT3 pathways. Brain Res. 2018, 1692, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Takata, F.; Matsumoto, J.; Takenoshita, H.; Kimura, I.; Yamauchi, A.; Dohgu, S.; Kataoka, Y. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood-brain barrier in vitro. Neurosci. Lett. 2015, 599, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Fruttiger, M.; D’Amore, P.; De Falco, S.; Joussen, A.; Sennlaub, F.; Brunck, L.; Johnson, K.; Lambrou, G.; Rittenhouse, K.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef] [PubMed]
- Hang, A.; Feldman, S.; Amin, A.; Ochoa, J.; Park, S. Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Retinal Disorders. Pharmaceuticals 2023, 16, 1140. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yun, J.; Kim, J.; Kim, K.; Cho, C.; Kim, J. Angiopoietin 2 induces pericyte apoptosis via α3β1 integrin signaling in diabetic retinopathy. Diabetes 2014, 63, 3057–3068. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Arimura, K.; Ago, T.; Kamouchi, M.; Nakamura, K.; Ishitsuka, K.; Kuroda, J.; Sugimori, H.; Ooboshi, H.; Sasaki, T.; Kitazono, T. PDGF receptor β signaling in pericytes following ischemic brain injury. Curr. Neurovascular Res. 2012, 9, 1–9. [Google Scholar] [CrossRef]
- Berrone, E.; Beltramo, E.; Buttiglieri, S.; Tarallo, S.; Rosso, A.; Hammes, H.P.; Porta, M. Establishment and characterization of a human retinal pericyte line: A novel tool for the study of diabetic retinopathy. Int. J. Mol. Med. 2009, 23, 373–378. [Google Scholar] [CrossRef] [PubMed]
- La Selva, M.; Beltramo, E.; Pagnozzi, F.; Bena, E.; Molinatti, P.A.; Molinatti, G.M.; Porta, M. Thiamine corrects delayed replication and decreases production of lactate and advanced glycation end-products in bovine retinal and human umbilical vein endothelial cells cultured under high glucose conditions. Diabetologia 1996, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Berrone, E.; Buttiglieri, S.; Porta, M. Thiamine and benfotiamine prevent increased apoptosis in endothelial cells and pericytes cultured in high glucose. Diabetes/Metab. Res. Rev. 2004, 20, 330–336. [Google Scholar] [CrossRef] [PubMed]



| ctrl | M1 | T | M1+T | |
|---|---|---|---|---|
| Size (nm) | 181.53 ± 13.23 | 175.48 ± 15.05 | 177.83 ± 9.43 | 183.08 ± 11.67 |
| Concentration (U × 1011/mL) | 3.04 ± 0.22 | 3.73 ± 0.41 | 2.71 ± 0.39 * | 2.74 ± 0.51 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltramo, E.; Mazzeo, A.; Porta, M. Release of Pro-Inflammatory/Angiogenic Factors by Retinal Microvascular Cells Is Mediated by Extracellular Vesicles Derived from M1-Activated Microglia. Int. J. Mol. Sci. 2024, 25, 15. https://doi.org/10.3390/ijms25010015
Beltramo E, Mazzeo A, Porta M. Release of Pro-Inflammatory/Angiogenic Factors by Retinal Microvascular Cells Is Mediated by Extracellular Vesicles Derived from M1-Activated Microglia. International Journal of Molecular Sciences. 2024; 25(1):15. https://doi.org/10.3390/ijms25010015
Chicago/Turabian StyleBeltramo, Elena, Aurora Mazzeo, and Massimo Porta. 2024. "Release of Pro-Inflammatory/Angiogenic Factors by Retinal Microvascular Cells Is Mediated by Extracellular Vesicles Derived from M1-Activated Microglia" International Journal of Molecular Sciences 25, no. 1: 15. https://doi.org/10.3390/ijms25010015
APA StyleBeltramo, E., Mazzeo, A., & Porta, M. (2024). Release of Pro-Inflammatory/Angiogenic Factors by Retinal Microvascular Cells Is Mediated by Extracellular Vesicles Derived from M1-Activated Microglia. International Journal of Molecular Sciences, 25(1), 15. https://doi.org/10.3390/ijms25010015