Overdue Calcium Oscillation Causes Polyspermy but Possibly Permits Normal Development in Mouse Eggs
Abstract
:1. Introduction
2. Results
2.1. Subcellular Localization of CS and eCS Proteins
2.2. Sperm Concentration and Ca2+ Oscillation Pattern
2.3. Sperm Concentration and Initiation of Ca2+ Oscillation
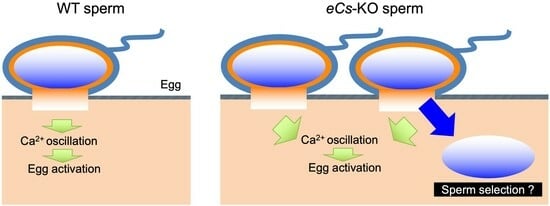
2.4. Multiple Fusion of eCs-KO Sperm with Zona-Free Eggs
2.5. Multiple Fusion of eCs-KO Sperm with Cumulus-Intact Eggs
2.6. Two-Cell Embryos and Blastocysts from Eggs Fertilized with eCs-KO Sperm
3. Discussion
3.1. Physiological Polyspermy
3.2. Mechanisms of Physiological Polyspermy
3.3. Pathological Polyspermy
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Transfection of Expression Vectors of CS and eCS Proteins
4.3. eCs-KO Mice
4.4. Measurement of Intracellular Ca2+ Concentration
4.5. In vitro Fertilization
4.6. Immunoblotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolf, J.P.; Ducot, B.; Aymar, C.; Rodrigues, D.; Desjardin, S.; Jardin, A.; Jouannet, P. Absence of block to polyspermy at the human oolemma. Fertil. Steril. 1997, 67, 1095–1102. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wang, Y.; Pan, J.; Liang, S.; Ruan, J.; Teng, X. Polarization microscopy imaging for the identification of unfertilized oocytes after short-term insemination. Fertil. Steril. 2017, 108, 78–83. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, H.; Chen, T.; Tian, Y.; Li, M.; Wu, K.; Bian, Y.; Su, S.; Cao, Y.; Ning, Y.; et al. Mutational analysis of IZUMO1R in women with fertilization failure and polyspermy after in vitro fertilization. J. Assist. Reprod. Genet. 2018, 35, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.L.; Bainbridge, R.E.; Summerville, D.W.; Tembo, M.; Phelps, W.A.; Sauer, M.L.; Wisner, B.W.; Czekalski, M.E.; Pasumarthy, S.; Hanson, M.L.; et al. Zinc protection of fertilized eggs is an ancient feature of sexual reproduction in animals. PLoS Biol. 2020, 18, e3000811. [Google Scholar] [CrossRef] [PubMed]
- Iwao, Y. Egg activation in physiological polyspermy. Reproduction 2012, 144, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Snook, R.R.; Hosken, D.J.; Karr, T.L. The biology and evolution of polyspermy: Insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction 2011, 142, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Harada, Y.; Yamatoya, K.; Kawano, N.; Kanai, S.; Miyamoto, Y.; Nakamura, A.; Miyado, M.; Hayashi, Y.; Kuroki, Y.; et al. Extra-mitochondrial citrate synthase initiates calcium oscillation and suppresses age-dependent sperm dysfunction. Lab. Investig. 2019, 100, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Lahbib-Mansais, Y.; Yerle, M.; Pinton, P.; Gellin, J. Chromosomal localization of homeobox genes and associated markers on porcine chromosomes 3, 5, 12, 15, 16 and 18: Comparative mapping study with human and mouse. Mamm Genome 1996, 7, 174–179. [Google Scholar] [CrossRef]
- Church, D.M.; Goodstadt, L.; Hillier, L.W.; Zody, M.C.; Goldstein, S.; She, X.; Bult, C.J.; Agarwala, R.; Cherry, J.L.; DiCuccio, M.; et al. Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biol. 2009, 7, e1000112. [Google Scholar] [CrossRef]
- Miyazaki, S. Early and later studies on action potential and fertilization potential of echinoderm oocytes and Ca(2+) response of mammalian oocytes. Methods Cell Biol. 2019, 151, 13–20. [Google Scholar]
- Harada, Y.; Matsumoto, T.; Hirahara, S.; Nakashima, A.; Ueno, S.; Oda, S.; Miyazaki, S.; Iwao, Y. Characterization of a sperm factor for egg activation at fertilization of the newt Cynops pyrrhogaster. Dev. Biol. 2007, 306, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; de Sutter, P.; et al. PLCzeta is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017, 144, 2914–2924. [Google Scholar] [PubMed]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, Y.; Deng, K.; Shen, J.; Cui, Y.; Liu, J.; Yang, X.; Diao, F. Mutations in PLCZ1 induce male infertility associated with polyspermy and fertilization failure. J. Assist. Reprod. Genet. 2023, 40, 53–64. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, H.H.; Al-Hasani, S.; Diedrich, K.; Hamerich, U.; Lehmann, F.; Krebs, D. Polyspermy in in vitro fertilization of human oocytes: Frequency and possible causes. Ann. N. Y Acad. Sci. 1985, 442, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, K.; Bai, D.; Yin, J.; Tang, Y.; Chi, F.; Zhang, L.; Wang, Y.; Pan, J.; Liang, S.; et al. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum. Genet. 2017, 136, 975–985. [Google Scholar] [CrossRef]
- Bhakta, H.H.; Refai, F.H.; Avella, M.A. The molecular mechanisms mediating mammalian fertilization. Development 2019, 146, dev176966. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef]
- Toyoda, Y.; Yokoyama, M.; Hosi, T. Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn. J. Anim. 1971, 16, 147–151. [Google Scholar]
- Torra-Massana, M.; Cornet-Bartolome, D.; Barragan, M.; Durban, M.; Ferrer-Vaquer, A.; Zambelli, F.; Rodriguez, A.; Oliva, R.; Vassena, R. Novel phospholipase C zeta 1 mutations associated with fertilization failures after ICSI. Hum. Reprod. 2019, 34, 1494–1504. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzene, T.; Coutton, C.; Kherraf, Z.E.; Halouani, L.; Triki, C.; et al. Homozygous mutation of PLCZ1 leads to defective human oocyte activation and infertility that is not rescued by the WW-binding protein PAWP. Hum. Mol. Genet. 2016, 25, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Fan, Y.; Wang, F.; Yan, Z.; Li, M.; Ouyang, J.; Wu, L.; Yin, M.; Zhao, J.; Kuang, Y.; et al. Novel mutations in PLCZ1 cause male infertility due to fertilization failure or poor fertilization. Hum. Reprod. 2020, 35, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, A. Correlation between the number of oocytes and the increase of polyspermy rate in IVF cycles. Gynecol. Endocrinol. 2023, 39, 2217270. [Google Scholar] [CrossRef]
- Yamatoya, K.; Ito, C.; Araki, M.; Furuse, R.; Toshimori, K. One-step collagenase method for zona pellucida removal in unfertilized eggs: Easy and gentle method for large-scale preparation. Reprod. Med. Biol. 2011, 10, 97–103. [Google Scholar] [CrossRef]
- Erbach, G.T.; Lawitts, J.A.; Papaioannou, V.E.; Biggers, J.D. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol. Reprod. 1994, 50, 1027–1033. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuoka, M.; Kang, W.; Katano, D.; Horiike, S.; Miyado, M.; Tanaka, M.; Miyado, K.; Yamada, M. Overdue Calcium Oscillation Causes Polyspermy but Possibly Permits Normal Development in Mouse Eggs. Int. J. Mol. Sci. 2024, 25, 285. https://doi.org/10.3390/ijms25010285
Fukuoka M, Kang W, Katano D, Horiike S, Miyado M, Tanaka M, Miyado K, Yamada M. Overdue Calcium Oscillation Causes Polyspermy but Possibly Permits Normal Development in Mouse Eggs. International Journal of Molecular Sciences. 2024; 25(1):285. https://doi.org/10.3390/ijms25010285
Chicago/Turabian StyleFukuoka, Mio, Woojin Kang, Daiki Katano, Sae Horiike, Mami Miyado, Mamoru Tanaka, Kenji Miyado, and Mitsutoshi Yamada. 2024. "Overdue Calcium Oscillation Causes Polyspermy but Possibly Permits Normal Development in Mouse Eggs" International Journal of Molecular Sciences 25, no. 1: 285. https://doi.org/10.3390/ijms25010285
APA StyleFukuoka, M., Kang, W., Katano, D., Horiike, S., Miyado, M., Tanaka, M., Miyado, K., & Yamada, M. (2024). Overdue Calcium Oscillation Causes Polyspermy but Possibly Permits Normal Development in Mouse Eggs. International Journal of Molecular Sciences, 25(1), 285. https://doi.org/10.3390/ijms25010285