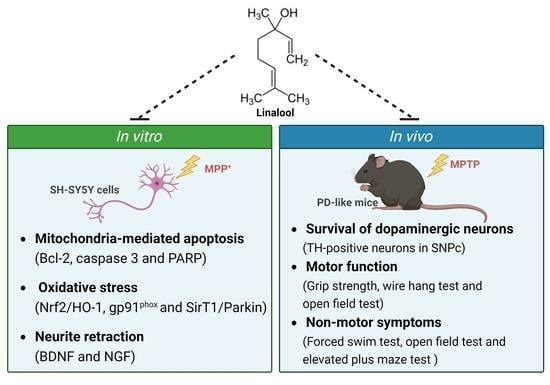
Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Linalool Increased the Cell Viability of MPP+-Treated SH-SY5Y Cells
2.2. Linalool Decreased MPP+-Induced Apoptotic Cell Death and Neurite Retraction Accompanied by Increasing Neurotrophic Factors in SH-SY5Y Cells
2.3. Linalool Enhanced Antioxidant Factors Nrf2/HO-1 and Inhibited NADPH Oxidase in MPP+-Treated SH-SY5Y Cells
2.4. Linalool Enhanced the Expression of SirT1, Parkin, and TH in MPP+-Treated Primary Mesencephalic Neurons and the Expression of SirT1 and γ-Aminobutyric Acid (GABA) in MPP+-Treated Primary Cortical Neurons
2.5. Linalool Protected Dopaminergic Neurons and Improved Motor Activity in MPTP-Induced Mouse Model of PD
2.6. Linalool Improved Depressive- and Anxiety-like Behaviors in the MPTP-Induced Mouse Model of PD
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. Apoptotic Nuclear Condensation
4.5. Measurement of Neurite Length
4.6. Western Blot Analysis
4.7. Animal Groupings and Drug Administration
4.8. Grip Strength Test
4.9. Wire Hang Test
4.10. Open-Field Test
4.11. Elevated plus Maze Test
4.12. Forced Swimming Test
4.13. Immunohistochemistry
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, H.C.; Liu, K.F.; Teng, C.J.; Lai, S.C.; Yang, S.E.; Ching, H.; Wu, C.R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients 2019, 11, 252. [Google Scholar] [CrossRef]
- Canet-Aviles, R.; Lomax, G.P.; Feigal, E.G.; Priest, C. Proceedings: Cell therapies for Parkinson’s disease from discovery to clinic. Stem Cells Transl. Med. 2014, 3, 979–991. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, Y.J.; Shin, M.S.; Kim, H.R.; Kim, M.J.; Lee, S.H.; Yun, S.P.; Kwon, S.H. Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson’s disease model. Bioorg. Med. Chem. Lett. 2017, 27, 5207–5212. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Schamne, M.G.; Mack, J.M.; Moretti, M.; Matheus, F.C.; Walz, R.; Lanfumey, L.; Prediger, R.D. The Gender-Biased Effects of Intranasal MPTP Administration on Anhedonic- and Depressive-Like Behaviors in C57BL/6 Mice: The Role of Neurotrophic Factors. Neurotox. Res. 2018, 34, 808–819. [Google Scholar] [CrossRef]
- Pont-Sunyer, C.; Hotter, A.; Gaig, C.; Seppi, K.; Compta, Y.; Katzenschlager, R.; Mas, N.; Hofeneder, D.; Brücke, T.; Bayés, A.; et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov. Disord. 2015, 30, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Grilo, A.L.; Mantalaris, A. Apoptosis: A mammalian cell bioprocessing perspective. Biotechnol. Adv. 2019, 37, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Alkholifi, F.K.; Devi, S.; Aldawsari, M.F.; Foudah, A.I.; Alqarni, M.H.; Salkini, M.A.; Sweilam, S.H. Effects of Tiliroside and Lisuride Co-Treatment on the PI3K/Akt Signal Pathway: Modulating Neuroinflammation and Apoptosis in Parkinson’s Disease. Biomedicines 2023, 11, 2735. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Gupta, V.; Chitranshi, N.; You, Y.; Dheer, Y.; Mirzaei, M.; Graham, S.L. Regulation of Brain-Derived Neurotrophic Factor and Growth Factor Signaling Pathways by Tyrosine Phosphatase Shp2 in the Retina: A Brief Review. Front. Cell. Neurosci. 2018, 12, 85. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; He, S.; Hong, B.; Peng, D.; Su, H.; Li, F.; Tang, Y.; Lin, Z.; Fang, Y.; et al. Nerve growth factor variations in patients with mood disorders: No changes in eight weeks of clinical treatment. Neuropsychiatr. Dis. Treat. 2014, 10, 835–840. [Google Scholar]
- Valvassori, S.S.; Mariot, E.; Varela, R.B.; Bavaresco, D.V.; Dal-Pont, G.C.; Ferreira, C.L.; Andersen, M.L.; Tye, S.J.; Quevedo, J. The role of neurotrophic factors in manic-, anxious- and depressive-like behaviors induced by amphetamine sensitization: Implications to the animal model of bipolar disorder. J. Affect. Disord. 2019, 245, 1106–1113. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Volcho, K.P.; Salakhutdinov, N.F. Neuroregeneration in Parkinson’s Disease: From Proteins to Small Molecules. Curr. Neuropharmacol. 2019, 17, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromolecular. Med. 2014, 16, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Johnson, J.A. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim. Biophys. Acta 2014, 1842, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Caramão, E.B.; Moreno, P.R.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Souto-Maior, F.N.; Fonsêca, D.V.; Salgado, P.R.; Monte, L.O.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive and anticonvulsant effects of the monoterpene linalool oxide. Pharm. Biol. 2017, 55, 63–67. [Google Scholar] [CrossRef]
- Azanchi, T.; Shafaroodi, H.; Asgarpanah, J. Anticonvulsant activity of Citrus aurantium blossom essential oil (neroli): Involvment of the GABAergic system. Nat. Prod. Commun. 2014, 9, 1615–1618. [Google Scholar] [PubMed]
- Berliocchi, L.; Russo, R.; Levato, A.; Fratto, V.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Mercuri, N.B.; Corasaniti, M.T. (-)-Linalool attenuates allodynia in neuropathic pain induced by spinal nerve ligation in c57/bl6 mice. Int. Rev. Neurobiol. 2009, 85, 221–235. [Google Scholar]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool Odor-Induced Anxiolytic Effects in Mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- Kitajima, M.; Miura, M.; Nanashima, N.; Tomisawa, T.; Takamagi, S.; Fujioka, M.; In, N.; Osanai, T. Psychological and Antibacterial Effects of Footbath Using the Lindera umbellata Essential Oil. Molecules 2021, 26, 5128. [Google Scholar] [CrossRef]
- Chang, H.T.; Chang, M.L.; Chen, Y.T.; Chang, S.T.; Hsu, F.L.; Wu, C.C.; Ho, C.K. Evaluation of Motor Coordination and Antidepressant Activities of Cinnamomum osmophloeum ct. Linalool Leaf Oil in Rodent Model. Molecules 2021, 26, 3037. [Google Scholar] [CrossRef]
- Homayoun, H. Parkinson Disease. Ann. Intern. Med. 2018, 169, Itc33–Itc48. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- de Lucena, J.D.; Gadelha-Filho, C.V.J.; da Costa, R.O.; de Araújo, D.P.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. L-linalool exerts a neuroprotective action on hemiparkinsonian rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1077–1088. [Google Scholar] [CrossRef]
- da Silva, P.R.; de Andrade, J.C.; de Sousa, N.F.; Ribeiro Portela, A.C.; Oliveira Pires, H.F.; Bezerra Remígio, M.C.R.; Alves, D.D.N.; de Andrade, H.H.N.; Dias, A.L.; da Silva Stiebbe Salvadori, M.G.; et al. Computational Studies Applied to Linalool and Citronellal Derivatives against Alzheimer’s and Parkinson’s Disorders: A Review with Experimental Approach. Curr. Neuropharmacol. 2023, 21, 842–866. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, S.T.; Liao, V.H. Potential anti-Parkinsonian’s effect of S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves are associated with mitochondrial regulation via gas-1, nuo-1, and mev-1 in Caenorhabditis elegans. Phytother. Res. 2022, 36, 3325–3334. [Google Scholar] [CrossRef]
- Munni, Y.A.; Dash, R.; Choi, H.J.; Mitra, S.; Hannan, M.A.; Mazumder, K.; Timalsina, B.; Moon, I.S. Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons. Int. J. Mol. Sci. 2023, 24, 13386. [Google Scholar] [CrossRef]
- Guzman-Gutierrez, S.L.; Bonilla-Jaime, H.; Gomez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef]
- Xu, P. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 7426538. [Google Scholar] [CrossRef]
- Chiueh, C.C.; Markey, S.P.; Burns, R.S.; Johannessen, J.N.; Jacobowitz, D.M.; Kopin, I.J. Neurochemical and behavioral effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in rat, guinea pig, and monkey. Psychopharmacol. Bull. 1984, 20, 548–553. [Google Scholar] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Shih, Y.T.; Tseng, Y.T.; Hsu, H.T. Neuroprotective Effects of San-Huang-Xie-Xin-Tang in the MPP+/MPTP Models of Parkinson’s Disease In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 501032. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, H.; Zhang, H.; Han, Y.; Yuan, J.; Wang, T.; Gao, Y.; Li, Z. Ameliorating Mitochondrial Dysfunction of Neurons by Biomimetic Targeting Nanoparticles Mediated Mitochondrial Biogenesis to Boost the Therapy of Parkinson’s Disease. Adv. Sci. 2023, 10, e2300758. [Google Scholar] [CrossRef]
- Tipton, K.F.; Singer, T.P. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 1993, 61, 1191–1206. [Google Scholar] [CrossRef]
- Narmashiri, A.; Abbaszadeh, M.; Ghazizadeh, A. The effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on the cognitive and motor functions in rodents: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 140, 104792. [Google Scholar] [CrossRef]
- Belarbi, K.; Cuvelier, E.; Destée, A.; Gressier, B.; Chartier-Harlin, M.C. NADPH oxidases in Parkinson’s disease: A systematic review. Mol. Neurodegener. 2017, 12, 84. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E. SIRT1 pathway in Parkinson’s disease: A faraway snapshot but so close. Inflammopharmacology 2023, 31, 37–56. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.S.; Won, S.Y.; Cho, K.S. Linalool Alleviates Aβ42-Induced Neurodegeneration via Suppressing ROS Production and Inflammation in Fly and Rat Models of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Chiavacci, E.; Bagnoli, S.; Cellerino, A.; Terzibasi Tozzini, E. Distribution of Brain-Derived Neurotrophic Factor in the Brain of the Small-Spotted Catshark Scyliorhinus canicula, and Evolution of Neurotrophins in Basal Vertebrates. Int. J. Mol. Sci. 2023, 24, 9495. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Di Monte, D.; Sandy, M.S.; Ekström, G.; Smith, M.T. Comparative studies on the mechanisms of paraquat and 1-methyl-4-phenylpyridine (MPP+) cytotoxicity. Biochem. Biophys. Res. Commun. 1986, 137, 303–309. [Google Scholar] [CrossRef]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural. Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef]
- Sorce, S.; Stocker, R.; Seredenina, T.; Holmdahl, R.; Aguzzi, A.; Chio, A.; Depaulis, A.; Heitz, F.; Olofsson, P.; Olsson, T.; et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: What is the evidence? Free Radic. Biol. Med. 2017, 112, 387–396. [Google Scholar] [CrossRef]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef]
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Ferrer, I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson’s disease. Park. Dis. 2011, 2011, 708404. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Folsom, T.D.; Rooney, R.J.; Thuras, P.D. Expression of GABAA α2-, β1- and ε-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl. Psychiatry 2013, 3, e303. [Google Scholar] [CrossRef] [PubMed]
- Möhler, H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Newton, D.; Lewis, D.; Sibille, E. Lower Levels of GABAergic Function Markers in Corticotropin-Releasing Hormone-Expressing Neurons in the sgACC of Human Subjects with Depression. Front. Psychiatry 2022, 13, 827972. [Google Scholar] [CrossRef]
- Luscher, B.; Maguire, J.L.; Rudolph, U.; Sibille, E. GABA(A) receptors as targets for treating affective and cognitive symptoms of depression. Trends Pharmacol. Sci. 2023, 44, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; Andikoetxea, A.; Gómez-Esteban, J.C.; Gabilondo, I. Contribution of the GABAergic System to Non-Motor Manifestations in Premotor and Early Stages of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tsai, C.W. PINK1/parkin-mediated mitophagy pathway is related to neuroprotection by carnosic acid in SH-SY5Y cells. Food Chem. Toxicol. 2019, 125, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Uchida, S.; Otsuki, K.; Hobara, T.; Yamagata, H.; Higuchi, F.; Shibata, T.; Watanabe, Y. Altered sirtuin deacetylase gene expression in patients with a mood disorder. J. Psychiatr. Res. 2011, 45, 1106–1112. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Misrani, A.; Huang, H.X.; Zhang, Z.Y.; Li, Q.W.; Long, C. Resveratrol Attenuates Chronic Unpredictable Mild Stress-Induced Alterations in the SIRT1/PGC1α/SIRT3 Pathway and Associated Mitochondrial Dysfunction in Mice. Mol. Neurobiol. 2023, 60, 5102–5116. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Hsu, Y.Y.; Shih, Y.T.; Lo, Y.C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock 2012, 37, 312–318. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wu, S.N.; Gao, J.M.; Liao, Z.Y.; Tseng, Y.T.; Fülöp, F.; Chang, F.R.; Lo, Y.C. The Antioxidant, Anti-Inflammatory, and Neuroprotective Properties of the Synthetic Chalcone Derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Chang, W.H.; Lin, C.C.; Chang, F.R.; Wu, P.C.; Lo, Y.C. Protective effects of Liuwei dihuang water extracts on diabetic muscle atrophy. Phytomedicine 2018, 53, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Yang, Y.L.; Chang, W.H.; Fang, W.Y.; Huang, S.H.; Chou, S.H.; Lo, Y.C. Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1alpha Pathway. Biomolecules 2022, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Seldeen, K.L.; Berman, R.N.; Pang, M.; Lasky, G.; Weiss, C.; MacDonald, B.A.; Thiyagarajan, R.; Redae, Y.; Troen, B.R. Vitamin D Insufficiency Reduces Grip Strength, Grip Endurance and Increases Frailty in Aged C57Bl/6J Mice. Nutrients 2020, 12, 3005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, F.; Gong, M.; Jin, L.; Ren, X.; Liu, K.; Lu, J. Lifelong treadmill training improves muscle function detected by a modified grip strength test during aging in BALB/c mice. Life Sci. 2020, 251, 117603. [Google Scholar] [CrossRef]
- Jo, M.G.; Ikram, M.; Jo, M.H.; Yoo, L.; Chung, K.C.; Nah, S.Y.; Hwang, H.; Rhim, H.; Kim, M.O. Gintonin Mitigates MPTP-Induced Loss of Nigrostriatal Dopaminergic Neurons and Accumulation of alpha-Synuclein via the Nrf2/HO-1 Pathway. Mol. Neurobiol. 2019, 56, 39–55. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Hua, X.; Zhang, H.; Sun, S.; Han, C. Three methods of behavioural testing to measure anxiety—A review. Behav. Process. 2024, 215, 104997. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-H.; Hsu, H.-T.; Lin, C.-C.; An, L.-M.; Lee, C.-H.; Ko, H.-H.; Lin, C.-L.; Lo, Y.-C. Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 2514. https://doi.org/10.3390/ijms25052514
Chang W-H, Hsu H-T, Lin C-C, An L-M, Lee C-H, Ko H-H, Lin C-L, Lo Y-C. Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease. International Journal of Molecular Sciences. 2024; 25(5):2514. https://doi.org/10.3390/ijms25052514
Chicago/Turabian StyleChang, Wan-Hsuan, Hung-Te Hsu, Chih-Cheng Lin, Li-Mei An, Chien-Hsing Lee, Horng-Huey Ko, Chih-Lung Lin, and Yi-Ching Lo. 2024. "Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease" International Journal of Molecular Sciences 25, no. 5: 2514. https://doi.org/10.3390/ijms25052514
APA StyleChang, W. -H., Hsu, H. -T., Lin, C. -C., An, L. -M., Lee, C. -H., Ko, H. -H., Lin, C. -L., & Lo, Y. -C. (2024). Linalool, a Fragrance Compound in Plants, Protects Dopaminergic Neurons and Improves Motor Function and Skeletal Muscle Strength in Experimental Models of Parkinson’s Disease. International Journal of Molecular Sciences, 25(5), 2514. https://doi.org/10.3390/ijms25052514