IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review
Abstract
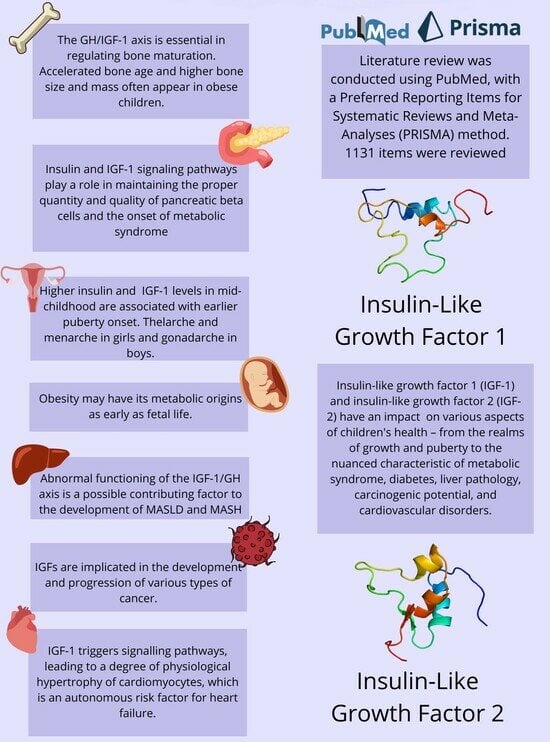
:1. Introduction
Family Physiology of IGFs
2. Methods





3. Results and Discussion
3.1. Impact of IGF-1 and IGF-2 on Child Growth
3.1.1. Fetal Life
3.1.2. Children’s Weight
3.1.3. Bone Development
3.1.4. Children’s Height and Its Relationship with Puberty
3.2. Impact of IGF-1 and IGF-2 on Puberty in Children
3.3. IGF-1 and IGF-2 in Metabolic Syndrome and Diabetes

3.4. The Influence of IGF-1 and IGF-2 on Hepatic Metabolism
3.5. IGF-1 and IGF-2 as Carcinogens and Cancer-Promoting Factors

3.6. The Role of IGF-1 and IGF-2 in Cardiovascular Disorders
3.7. Discussion
4. Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and adolescent obesity. Nat. Rev. Dis. Primers 2023, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Czogała, W.; Strojny, W.; Tomasik, P.; Bik-Multanowski, M.; Wójcik, M.; Miklusiak, K.; Krzysztofik, E.; Wróbel, A.; Miklusiak, K.; Skoczeń, S. The Insight into Insulin-Like Growth Factors and Insulin-Like Growth-Factor-Binding Proteins and Metabolic Profile in Pediatric Obesity. Nutrients 2021, 13, 2432. [Google Scholar] [CrossRef] [PubMed]
- Kempf, E.; Landgraf, K.; Vogel, T.; Spielau, U.; Stein, R.; Raschpichler, M.; Kratzsch, J.; Kieß, W.; Staník, J.; Körner, A. Associations of GHR, IGF-1 and IGFBP-3 expression in adipose tissue cells with obesity-related alterations in corresponding circulating levels and adipose tissue function in children. Adipocyte 2022, 11, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Inzaghi, E.; Ferroli, B.B.; Fintini, D.; Grossi, A.; Nobili, V.; Cianfarani, S. Insulin-Like growth factors and metabolic syndrome in obese children. Horm. Res. Paediatr. 2017, 87, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhao, J.; Wu, Y.; Zhao, Y.; Chen, C.; Xu, M.; Cheng, J.; Li, C. Association between insulin-like growth factor 1 gene rs35767 polymorphisms and cancer risk: A meta-analysis. Medicine 2019, 98, e18017. [Google Scholar] [CrossRef] [PubMed]
- Vishwamitra, D.; George, S.K.; Shi, P.; Kaseb, A.O.; Amin, H.M. Type I insulin-like growth factor receptor signaling in hematological malignancies. Oncotarget 2017, 8, 1814–1844. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Rogol, A.D.; Rosenfeld, R.G. The history of the Insulin-Like Growth Factor System. Horm. Res. Paediatr. 2022, 95, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Holly, J.M.P.; Biernacka, K.; Perks, C.M. The Neglected Insulin: IGF-II, a Metabolic Regulator with Implications for Diabetes, Obesity, and Cancer. Cells 2019, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Bang, P. Pediatric Implications of Normal Insulin-GH-IGF-Axis Physiology. In Endotext; Feingold, K.R., Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Weng, X.F.; Huang, B.L.; Guo, H.P.; Xu, Y.W.; Peng, Y.H. IGFBP-1 in cancer: Expression, molecular mechanisms, and potential clinical implications. Am. J. Transl. Res. 2021, 13, 813–832. [Google Scholar] [PubMed]
- Boughanem, H.; Yubero-Serrano, E.M.; López-Miranda, J.; Tinahones, F.J.; Macías-González, M. Potential role of Insulin Growth-Factor-Binding Protein 2 as therapeutic target for Obesity-Related insulin resistance. Int. J. Mol. Sci. 2021, 22, 1133. [Google Scholar] [CrossRef]
- Scully, T.; Firth, S.M.; Scott, C.D.; De Silva, H.C.; Pintar, J.E.; Chan-Ling, T.; Twigg, S.M.; Baxter, R.C. Insulin-like growth factor binding protein-3 links obesity and breast cancer progression. Oncotarget 2016, 7, 55491–55505. [Google Scholar] [CrossRef]
- Nur, S.I.; Öztürk, A.; Kavas, M.; Bulut, İ.; Alparslan, S.; Aydogan, E.S.; Atinkaya, C.; Kolay, M.; Coşkun, A. IGFBP-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Allard, J.B. Insulin-Like growth Factor binding protein-5 in physiology and disease. Front. Endocrinol. 2020, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Liso, A.; Venuto, S.; Coda, A.R.D.; Giallongo, C.; Palumbo, G.A.; Tibullo, D. IGFBP-6: At the crossroads of immunity, tissue repair and fibrosis. Int. J. Mol. Sci. 2022, 23, 4358. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Perks, C.M. Editorial: The role of the IGF/Insulin-IGFBP Axis in Normal Physiology and Disease. Front. Endocrinol. 2022, 13, 892140. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Klein, K.O.; Newfield, R.S.; Hassink, S.G. Bone maturation along the spectrum from normal weight to obesity: A complex interplay of sex, growth factors and weight gain. J. Pediatr. Endocrinol. Metab. 2016, 29, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, S.; Gat-Yablonski, G. Associations of Obesity with Linear Growth and Puberty. Horm. Res. Paediatr. 2022, 95, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Dalskov, S.; Ritz, C.; Larnkjær, A.; Damsgaard, C.T.; Petersen, R.A.; Sørensen, L.B.; Ong, K.K.; Astrup, A.; Michaelsen, K.F.; Mølgaard, C. Associations between adiposity, hormones, and gains in height, whole-body height-adjusted bone size, and size-adjusted bone mineral content in 8- to 11-year-old children. Osteoporos. Int. 2016, 27, 1619–1629. [Google Scholar] [CrossRef]
- Keleher, M.R.; Erickson, K.; Smith, H.A.; Kechris, K.J.; Yang, I.V.; Dabelea, D.; Friedman, J.E.; Boyle, K.E.; Jansson, T. Placental Insulin/IGF-1 Signaling, PGC-1α, and Inflammatory Pathways Are Associated with Metabolic Outcomes at 4–6 Years of Age: The ECHO Healthy Start Cohort. Diabetes 2021, 70, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.; Josefson, J. The Relationship of Insulin-Like Growth Factor 2 to Fetal Growth and Adiposity. Horm. Res. Paediatr. 2016, 85, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Larnkjaer, A.; Ong, K.K.; Carlsen, E.M.; Ejlerskov, K.T.; Molgaard, C.; Michaelsen, K.F. The Influence of Maternal Obesity and Breastfeeding on Infant Appetite- and Growth-Related Hormone Concentrations: The SKOT Cohort Studies. Horm. Res. Paediatr. 2018, 90, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.C.; Cleves, M.A.; DiCarlo, M.; Sobik, S.R.; Ruebel, M.L.; Thakali, K.M.; Sims, C.R.; Dajani, N.K.; Krukowski, R.A.; Børsheim, E.; et al. Parental adiposity differentially associates with newborn body composition. Pediatr. Obes. 2020, 15, e12596. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, M.; Murray, D.M.; Hawkes, C.P.; Kiely, M.; Ní Chaoimh, C.; McCarthy, F.P.; Biesma, R.; Boland, F. Body Mass Index Trajectories in the First 5 Years and Associated Antenatal Factors. Front. Pediatr. 2021, 9, 622381. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hellmuth, C.; Uhl, O.; Godfrey, K.; Briley, A.; Welsh, P.; Pasupathy, D.; Seed, P.T.; Koletzko, B.; Poston, L.; et al. Cord metabolic profiles in obese pregnant women: Insights into offspring growth and body composition. J. Clin. Endocrinol. Metab. 2018, 103, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Hellmuth, C.; Gruszfeld, D.; Demmelmair, H.; Rzehak, P.; Grote, V.; Weber, M.; Escribano, J.; Closa-Monasterolo, R.; Dain, E.; et al. European Childhood Obesity Trial Study Group. Endocrine and Metabolic Biomarkers Predicting Early Childhood Obesity Risk. Nestle Nutr. Inst. Workshop Ser. 2016, 85, 81–88. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Gupta, S.; Khurana, A.K.; Narang, A.; Shukla, M.; Arora, P.; Chadha, A.; Nayak, D.D.; Manchanda, R.K. Assessment of insulin like growth factor-1 and IGF binding protein-3 in healthy Indian girls from Delhi and their correlation with age, pubertal status, obesity and thyroid hormonal status. J. Pediatr. Endocrinol. Metab. 2017, 30, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hu, Y.; Liu, C.; Qi, J.; Li, G. Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: A cross-sectional study. Lipids Health Dis. 2016, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yu, Z.; Song, X.; Wang, Y.; Li, M.; Xue, J. Reduced Growth Hormone Secretion is Associated with Nonalcoholic Fatty Liver Disease in Obese Children. Horm. Metab. Res. 2018, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, D.; Qi, J.; Song, X.; Xue, J. Reduced peak stimulated growth hormone is associated with hyperuricemia in obese children and adolescents. Sci. Rep. 2018, 8, 7931. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.M.; Ortiz, A.P.; Fuentes-Mattei, E.; Velázquez-Torres, G.; Santiago, D.; Giovannetti, K.; Bernabe, R.; Lee, M.H.; Yeung, S.C. High prevalence of cardiometabolic risk factors in Hispanic adolescents: Correlations with adipocytokines and markers of inflammation. J. Immigr. Minor. Health 2014, 16, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Hörenz, C.; Vogel, M.; Wirkner, K.; Ceglarek, U.; Thiery, J.; Pfäffle, R.; Kiess, W.; Kratzsch, J. BMI and Contraceptives Affect New Age-, Sex-, and Puberty-adjusted IGF-I and IGFBP-3 Reference Ranges Across Life Span. J. Clin. Endocrinol. Metab. 2022, 107, e2991–e3002. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, M.; Martinovic, M.; Gligorovic-Barhanovic, N.; Antunovic, T.; Nedovic-Vukovic, M. Relationship between insulin-like growth factor-1, insulin resistance and metabolic profile with pre-obesity and obesity in children. J. Pediatr. Endocrinol. Metab. 2021, 34, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Vergani, E.; Bruno, C.; Cipolla, C.; Currò, D.; Mancini, A. Plasma Levels of Neudesin and Glucose Metabolism in Obese and Overweight Children. Front. Endocrinol. 2022, 13, 881524. [Google Scholar] [CrossRef] [PubMed]
- Mesa Valencia, D.C.; Mericq, V.; Corvalán, C.; Pereira, A. Obesity and Related Metabolic Biomarkers and Its Association with Serum Levels of Estrogen in Pre-pubertal Chilean Girls. Endocr. Res. 2020, 45, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Martínez Cuevas, E.; Muñoz Peláez, C.; Ordax Carbajo, E.; Navazo Eguia, A.I.; Martín Viñe, L.; Prieto Jimeno, A.; Alonso-Álvarez, M.L. Sleep apnoea-hypopnoea syndrome in the obese and non-obese: Clinical, polysomnographical and clinical characteristics. An. Pediatr. (Engl. Ed.) 2021, 95, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zong, X.; Zhang, Y.; Guo, J.; Li, H. Association of Body Mass Index with Insulin-like Growth Factor-1 Levels among 3227 Chinese Children Aged 2–18 Years. Nutrients 2023, 15, 1849. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Klemarczyk, W.; Ambroszkiewicz, J.; Szamotulska, K.; Chełchowska, M.; Weker, H. Associations between IGF-I, IGF-binding proteins and bone turnover markers in prepubertal obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Stawerska, R.; Czkwianianc, E.; Smyczyńska, J.; Hilczer, M.; Lewiński, A. Nutritional Status in Short Stature Children Is Related to Both Ghrelin and Insulin-like Growth Factor I Concentrations. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Ejlerskov, K.T.; Larnkjaer, A.; Pedersen, D.; Ritz, C.; Mølgaard, C.; Michaelsen, K.F. IGF-I at 9 and 36 months of age—relations with body composition and diet at 3 years—the SKOT cohort. Growth Horm. IGF Res. 2014, 24, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Damsgaard, C.T.; Harsløf, L.B.; Andersen, A.D.; Hellgren, L.I.; Michaelsen, K.F.; Lauritzen, L. Fish oil supplementation from 9 to 18 months of age affects the insulin-like growth factor axis in a sex-specific manner in Danish infants. Br. J. Nutr. 2016, 115, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, R.; Solito, A.; Mariotti Zani, E.; Caputo, M.; Genoni, G.; Barone-Adesi, F.; Mancioppi, V.; Agosti, E.; Aimaretti, G.; Bellone, S.; et al. The relationship between cortisol and IGF-I influences metabolic alteration in pediatric overweight and obesity. Eur. J. Endocrinol. 2020, 182, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Karaoglan, M.; Balci, O.; Keskin, M. Changes in thyroid volume and insulin-like growth factor 1 in pre- and post-pubertal obese children. Niger. J. Clin. Pract. 2019, 22, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, P.; Zenzeri, L.; Mozzillo, E.; Giorgio, V.; Rocco, A.; Franzese, A.; Nardone, G.; Staiano, A. Plasma dosage of ghrelin, IGF-1, GLP-1 and leptin related to gastric emptying and esophageal pH-impedance in children with obesity. J. Endocrinol. Investig. 2021, 44, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.E.; Gralewski, K.A.; Abrams, P.; Brar, P.C.; Gallagher, P.R.; Lipman, T.H.; Brooks, L.J.; Koren, D. Insulin-like growth factor-I and insulin-like growth factor binding protein-1 are related to cardiovascular disease biomarkers in obese adolescents. Pediatr. Diabetes 2016, 17, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mericq, V.; Pereira, A.; Uauy, R.; Corvalán, C. Early BMI Gain and Later Height Growth Predicts Higher DHEAS Concentrations in 7-Year-Old Chilean Children. Horm. Res. Paediatr. 2017, 87, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Su, Z.; Pan, L.; Wang, L.; Xu, Z.; Peng, G.; Li, X. Factors affecting bone maturation in Chinese girls aged 4–8 years with isolated premature thelarche. BMC Pediatr. 2020, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; De Sanctis, V.; Elalaily, R. Nutrition and pubertal development. Indian. J. Endocrinol. Metab. 2014, 18 (Suppl. S1), S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Roth, C.L.; Woelfle, J. Fibroblast growth factor 21 (FGF-21) in obese children: No relationship to growth, IGF-1, and IGFBP-3. Horm. Mol. Biol. Clin. Investig. 2017, 30, 20150074. [Google Scholar] [CrossRef] [PubMed]
- Baier, I.; Pereira, A.; Ferrer, P.; Iñiguez, G.; Mericq, V. Higher Prepubertal IGF-1 Concentrations Associate to Earlier Pubertal Tempo in Both Sexes. Horm. Res. Paediatr. 2023, 96, 404–411. [Google Scholar] [CrossRef]
- Kempf, E.; Vogel, M.; Vogel, T.; Kratzsch, J.; Landgraf, K.; Kühnapfel, A.; Gausche, R.; Gräfe, D.; Sergeyev, E.; Pfäffle, R.; et al. Dynamic alterations in linear growth and endocrine parameters in children with obesity and height reference values. EClinicalMedicine 2021, 37, 100977. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi, L.; Favilla, V.; Morgia, G.; Cimino, S.; Russo, G.; La Vignera, S. The gonadal function in obese adolescents: Review. J. Endocrinol. Investig. 2014, 37, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Vergani, E.; Giusti, M.; Oliva, A.; Cipolla, C.; Pitocco, D.; Mancini, A. The “Adipo-Cerebral” Dialogue in Childhood Obesity: Focus on Growth and Puberty. Physiopathological and Nutritional Aspects. Nutrients 2021, 13, 3434. [Google Scholar] [CrossRef]
- Silva, M.A.F.S.; Dechichi, P.; Limirio, P.H.J.O. Impact of Childhood Obesity on Bone Metabolism. Pediatr. Endocrinol. Rev. 2020, 17, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Bosse, C.; Lass, N.; Rothermel, J.; Knop, C.; Roth, C.L. Effect of Weight Loss on Puberty Onset in Overweight Children. J. Pediatr. 2017, 184, 143–150.e1. [Google Scholar] [CrossRef]
- Meln, I.; Wolff, G.; Gajek, T.; Koddebusch, J.; Lerch, S.; Harbrecht, L.; Hong, W.; Bayindir-Buchhalter, I.; Krunic, D.; Augustin, H.G.; et al. Dietary calories and lipids synergistically shape adipose tissue cellularity during postnatal growth. Mol. Metab. 2019, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, C.; Pedicelli, S.; Romanzo, A.; Bocchini, S.; Bottaro, G.; Cianfarani, S.; Cappa, M. The impact of IGF-I, puberty and obesity on early retinopathy in children: A cross-sectional study. Ital. J. Pediatr. 2019, 45, 52. [Google Scholar] [CrossRef]
- Haywood, N.; Slater, T.A.; Matthews, C.; Wheatcroft, S. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Anam, A.K.; Cooke, K.; Dratver, M.B.; O’Bryan, J.V.; Perley, L.; Guller, S.; Hwang, J.; Taylor, H.S.; Goedeke, L.; Kliman, H.J.; et al. Insulin increases placental triglyceride as a potential mechanism for fetal adiposity in maternal obesity. Mol. Metab. 2022, 64, 101574. [Google Scholar] [CrossRef] [PubMed]
- Łupińska, A.; Stawerska, R.; Szałapska, M.; Kolasa-Kicińska, M.; Jeziorny, K.; Stawerski, W.; Aszkiełowicz, S.; Lewiński, A. The incidence of insulin resistance based on indices calculated using the HOMA and Belfiore methods and its impact on the occurrence of metabolic complications in prepubertal children born small for gestational age. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E.; Corey, K.E.; Haines, M.S.; Chicote, M.L.; Kimball, A.; Colling, C.; Simon, T.G.; Long, M.T.; Husseini, J.S.; Bredella, M.A.; et al. The GH/IGF-1 axis is associated with intrahepatic lipid content and hepatocellular damage in Overweight/Obesity. J. Clin. Endocrinol. Metab. 2022, 107, e3624–e3632. [Google Scholar] [CrossRef]
- Marques, V.; Afonso, M.B.; Bierig, N.; Duarte-Ramos, F.; Santos-Laso, Á.; Jimenez-Agüero, R.; Eizaguirre, E.; Bujanda, L.; Pareja, M.J.; Luís, R.; et al. Adiponectin, leptin, and IGF-1 are useful diagnostic and stratification biomarkers of NAFLD. Front. Med. 2021, 8, 683250. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, E.; Rinaldi, L.; Mormone, A.; Giorgione, C.; Galiero, R.; Caturano, A.; Nevola, R.; Marfella, R.; Sasso, F.C. Non-alcoholic fatty liver disease (NAFLD), Type 2 diabetes, and non-viral hepatocarcinoma: Pathophysiological mechanisms and new therapeutic strategies. Biomedicines 2023, 11, 468. [Google Scholar] [CrossRef]
- Scalera, A.; Tarantino, G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J. Gastroenterol. 2014, 20, 9217–9228. [Google Scholar] [PubMed]
- Takahashi, Y. The role of growth hormone and Insulin-Like growth Factor-I in the liver. Int. J. Mol. Sci. 2017, 18, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Mohaqiq, M.; Shoorei, H.; Taheri, M. The interplay between non-coding RNAs and Insulin-Like growth factor signaling in the pathogenesis of neoplasia. Front. Cell Dev. Biol. 2021, 9, 634512. [Google Scholar] [CrossRef] [PubMed]
- Ingrid, L.; Stanley, T. Growth hormone and nonalcoholic fatty liver disease. Immunometabolism 2023, 5, e00030. [Google Scholar] [CrossRef]
- Chishima, S.; Kogiso, T.; Matsushita, N.; Hashimoto, E.; Tokushige, K. The Relationship between the Growth Hormone/Insulin-like Growth Factor System and the Histological Features of Nonalcoholic Fatty Liver Disease. Intern. Med. 2017, 56, 473–480. [Google Scholar] [CrossRef]
- Runchey, S.; Boyko, E.J.; Ioannou, G.N.; Utzschneider, K.M. Relationship between serum circulating insulin-like growth factor-1 and liver fat in the United States. J. Gastroenterol. Hepatol. 2014, 29, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.J.C.M.M.; Van Der Lely, A.-J. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.; Fourman, L.T.; Zheng, I.; McClure, C.M.; Feldpausch, M.N.; Torriani, M.; Corey, K.E.; Chung, R.T.; Lee, H.; Kleiner, D.E.; et al. Relationship of IGF-1 and IGF-Binding proteins to disease severity and glycemia in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2020, 106, e520–e533. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Sellers, J.; Smith, S.; Bhogoju, S.; Junkins, S.; Welch, M.; Willoughby, O.S.; Ghimire, N.; Secunda, C.; Barmanova, M.; et al. Metabolic impact of MKP-2 upregulation in obesity promotes insulin resistance and fatty liver disease. Nutrients 2022, 14, 2475. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, J.; Yu, R. Newly discovered endocrine functions of the liver. World J. Hepatol. 2021, 13, 1611–1628. [Google Scholar] [CrossRef] [PubMed]
- De La Garza, R.G.; Morales-Garza, L.A.; Martín-Estal, I.; Castilla-Cortázar, I. Insulin-Like growth factor-1 deficiency and cirrhosis establishment. J. Clin. Med. Res. 2017, 9, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Dichtel, L.E.; Corey, K.E.; Misdraji, J.; Bredella, M.A.; Schorr, M.; Osganian, S.A.; Young, B.J.; Sung, J.; Miller, K.K. The association between IGF-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin. Transl. Gastroenterol. 2017, 8, e217. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Lubrano, C.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Fatty Liver Index Associates with Relative Sarcopenia and GH/ IGF- 1 Status in Obese Subjects. PLoS ONE 2016, 11, e0145811. [Google Scholar] [CrossRef] [PubMed]
- Adamek, A.; Kasprzak, A. Insulin-Like growth Factor (IGF) system in liver diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef]
- Prunier, C.; Prudent, R.; Kapur, R.; Sadoul, K.; Lafanéchère, L. Oncotarget. Oncotarget 2017, 8, 41749–41763. [Google Scholar] [CrossRef] [PubMed]
- Elmashad, N.; Ibrahim, W.A.; Mayah, W.; Farouk, M.; Ali, L.A.; Taha, A.; Elmashad, W. Predictive value of serum insulin-like growth factor-1 in hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Perakakis, N.; Boutari, C.; Ghaly, W.; Anastasilakis, A.D.; Karagiannis, A.; Mantzoros, C.S. Targeted Analysis of Three Hormonal Systems Identifies Molecules Associated with the Presence and Severity of NAFLD. J. Clin. Endocrinol. Metab. 2019, 105, e390–e400. [Google Scholar] [CrossRef]
- Del Rocío Ibarra-Reynoso, L.; Pisarchyk, L.; Pérez-Luque, E.; Garay-Sevilla, M.E.; Malacara, J.M. Whole-Body and hepatic insulin resistance in obese children. PLoS ONE 2014, 9, e113576. [Google Scholar] [CrossRef]
- Deal, B.J.; Huffman, M.D.; Binns, H.; Stone, N.J. Perspective: Childhood Obesity Requires New Strategies for Prevention. Adv. Nutr. 2020, 11, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Kim, G. Role of physical exercise in modulating the insulin-like growth factor system for improving breast cancer outcomes: A meta-analysis. Exp. Gerontol. 2021, 152, 111435. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Baczmańska, D.; Malinowski, A.; Głowacka, E.; Wilczyński, M. Does IGF-1 play a role in the biology of ovarian cancer? Ginekol. Pol. 2018, 89, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, T.U.; Peisch, S.; Pettersson, A.; Ebot, E.M.; Zhou, C.K.; Graff, R.E.; Sinnott, J.A.; Fazli, L.; Judson, G.L.; Bismar, T.A.; et al. Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis 2018, 39, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Jain, N.; Ferrajoli, A.; O’Brien, S.; Burger, J.; Keating, M.; Wierda, W. A phase II trial of eltrombopag for patients with chronic lymphocytic leukaemia (CLL) and thrombocytopenia. Br. J. Haematol. 2019, 185, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, D.; Zaramella, A.; Fabris, F.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Insulin/IGF-1 Signaling Is Downregulated in Barrett’s Esophagus Patients Undergoing a Moderate Calorie and Protein Restriction Program: A Randomized 2-Year Trial. Nutrients 2021, 13, 3638. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, D.; Dedja, A.; Giacometti, C.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Hyperinsulinemia Promotes Esophageal Cancer Development in a Surgically-Induced Duodeno-Esophageal Reflux Murine Model. Int. J. Mol. Sci. 2018, 19, 1198. [Google Scholar] [CrossRef] [PubMed]
- Scalia, P.; Giordano, A.; Williams, S.J. The IGF-II-Insulin Receptor Isoform-A Autocrine Signal in Cancer: Actionable Perspectives. Cancers 2020, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; Goonathilake, M.R.; Waqar, S.; et al. The Role of Type 2 Diabetes in Pancreatic Cancer. Cureus 2022, 14, e26288. [Google Scholar] [CrossRef]
- Toriola, A.T.; Ziegler, M.; Li, Y.; Pollak, M.; Stolzenberg-Solomon, R. Prediagnosis Circulating Insulin-Like Growth Factors and Pancreatic Cancer Survival. Ann. Surg. Oncol. 2017, 24, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.W.; Fontaine, K.R.; Arend, R.C.; Alvarez, R.D.; Leath, C.A., III; Huh, W.K.; Bevis, K.S.; Kim, K.H.; Straughn, J.M., Jr.; Gower, B.A. A Ketogenic Diet Reduces Central Obesity and Serum Insulin in Women with Ovarian or Endometrial Cancer. J. Nutr. 2018, 148, 1253–1260. [Google Scholar] [CrossRef]
- Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, R.; Sienkiewicz, A.; Kinalski, M.; Terlikowski, S.J. Clinical value of selected markers of angiogenesis, inflammation, insulin resistance and obesity in type 1 endometrial cancer. BMC Cancer 2020, 20, 921. [Google Scholar] [CrossRef]
- Demetriou, E.; Fokou, M.; Frangos, S.; Papageorgis, P.; Economides, P.A.; Economides, A. Thyroid Nodules and Obesity. Life 2023, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Gluba-Brzózka, A. The Role of Metabolic Factors in Renal Cancers. Int. J. Mol. Sci. 2020, 21, 7246. [Google Scholar] [CrossRef]
- Sun, L.; Gao, Z.; Luo, L.; Tan, H.; Zhang, G. Estrogen affects cell growth and IGF-1 receptor expression in renal cell carcinoma. Onco Targets Ther. 2018, 11, 5873–5878. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chao, F.; Luo, B.; Ye, D.; Zhao, J.; Zhang, Q.; Ma, X.; Zhang, G. Impact of Estrogen on the Relationship Between Obesity and Renal Cell Carcinoma Risk in Women. EBioMedicine 2018, 34, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, H.; Kutbi, E.H.; Tan, S.C.; Low, T.Y.; Zhang, C. Association of IGF-1 and IGFBP-3 levels with gastric cancer: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14764. [Google Scholar] [CrossRef]
- Mohebi, R.; Liu, Y.; Hansen, M.K.; Yavin, Y.; Sattar, N.; Pollock, C.A.; Butler, J.; Jardine, M.; Masson, S.; Heerspink, H.J.L.; et al. Insulin growth factor axis and cardio-renal risk in diabetic kidney disease: An analysis from the CREDENCE trial. Cardiovasc. Diabetol. 2023, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.P.C.; Senger, N.; Barreto-Chaves, M.L.M. The endocrinological component and signaling pathways associated to cardiac hypertrophy. Mol. Cell Endocrinol. 2020, 518, 110972. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Gautam, S.; Delafontaine, P.; Sukhanov, S. IGF-1 and cardiovascular disease. Growth Horm. IGF Res. 2019, 45, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Mosca, S.; Paolillo, S.; Colao, A.; Bossone, E.; Cittadini, A.; Iudice, F.L.; Parente, A.; Conte, S.; Rengo, G.; Leosco, D.; et al. Cardiovascular involvement in patients affected by acromegaly: An appraisal. Int. J. Cardiol. 2013, 167, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Grasso, L.F.S.; Di Somma, C.; Pivonello, R. Acromegaly and Heart Failure. Heart Fail. Clin. 2019, 15, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, L.; Song, B.; Cui, Z.; Chen, G.; Yu, Z.; Song, B. Insulin-like Growth Factor-2 (IGF-2) in Fibrosis. Biomolecules 2022, 12, 1557. [Google Scholar] [CrossRef]

| Paragraph | Search Terms |
|---|---|
| Impact of IGF-1 and IGF-2 on children’s growth (Figure 1) | child* OR pediatric* OR paediatric* AND obes* AND IGF-1 OR IGF-2 OR IGF |
| IGF-1 and IGF-2 in metabolic syndrome and diabetes (Figure 2) | child* OR “pediatric* OR paediatric* OR fetal OR baby AND obes* AND IGF-1 OR IGF-2 OR IGF AND diabetes OR metabolic syndrome NOT diabetes type 1 |
| The influence of IGF-1 and IGF-2 on hepatic metabolism (Figure 3) | child* OR pediatric* OR paediatric* AND obes* AND IGF-1 OR IGF-2 OR IGF AND liver disease OR cirrhosis OR liver damage OR fatty liver |
| IGF-1 and IGF-2 as carcinogens and cancer- promoting factors (Figure 4) | obes* AND IGF-1 OR IGF-2 OR IGF AND cancer OR carcinogens OR cancer promoting agent* OR Chronic Lymphocytic Leukemia OR carcinogenesis OR tumor |
| The role of IGF-1 and IGF-2 in cardiovascular disorders (Figure 5) | obes* AND IGF-1 OR IGF-2 OR IGF AND cardiovascular disorder* OR heart failure OR cardiac hypertrophy OR cardiomyocyte* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydlowska-Gladysz, J.; Gorecka, A.E.; Stepien, J.; Rysz, I.; Ben-Skowronek, I. IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3966. https://doi.org/10.3390/ijms25073966
Szydlowska-Gladysz J, Gorecka AE, Stepien J, Rysz I, Ben-Skowronek I. IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(7):3966. https://doi.org/10.3390/ijms25073966
Chicago/Turabian StyleSzydlowska-Gladysz, Justyna, Adrianna Edyta Gorecka, Julia Stepien, Izabela Rysz, and Iwona Ben-Skowronek. 2024. "IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review" International Journal of Molecular Sciences 25, no. 7: 3966. https://doi.org/10.3390/ijms25073966
APA StyleSzydlowska-Gladysz, J., Gorecka, A. E., Stepien, J., Rysz, I., & Ben-Skowronek, I. (2024). IGF-1 and IGF-2 as Molecules Linked to Causes and Consequences of Obesity from Fetal Life to Adulthood: A Systematic Review. International Journal of Molecular Sciences, 25(7), 3966. https://doi.org/10.3390/ijms25073966