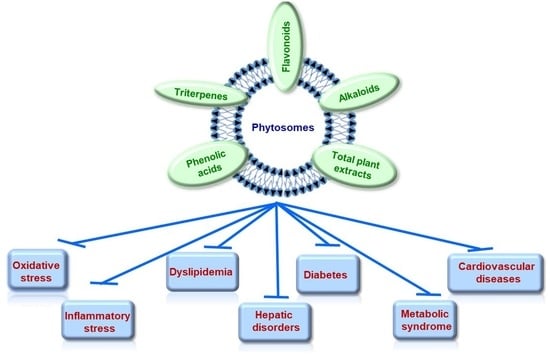
Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders
Abstract
:1. Introduction
2. Preparation and Characterization of Phytosomes with Bioactive Compounds
2.1. Preparation of Phytosomes with Bioactive Compounds
2.2. Characterization of Phytosomes with Bioactive Compounds
3. Evaluation of the Bioavailability of Active Compounds Formulated in Phytosomes
4. Pathologies Addressed by Natural Bioactive Compounds Formulated in Phytosomes
4.1. Oxidative and Inflammatory Stress
4.1.1. Preclinical Studies
4.1.2. Clinical Studies
4.2. Dyslipidemia
4.2.1. Preclinical Studies
4.2.2. Clinical Studies
4.3. Hepatic Disorders
4.3.1. Preclinical Studies
4.3.2. Clinical Studies
4.4. Diabetes Mellitus
4.4.1. Preclinical Studies
4.4.2. Clinical Studies
4.5. Metabolic Syndrome
4.5.1. Preclinical Studies
4.5.2. Clinical Studies
4.6. Cardiovascular Disorders
4.6.1. Preclinical Studies
4.6.2. Clinical Studies
5. Final Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brønden, A.; Christensen, M.B.; Glintborg, D.; Snorgaard, O.; Kofoed-Enevoldsen, A.; Madsen, G.K.; Toft, K.; Kristensen, J.K.; Højlund, K.; Hansen, T.K.; et al. Effects of DPP-4 inhibitors, GLP-1 receptor agonists, SGLT-2 inhibitors and sulphonylureas on mortality, cardiovascular and renal outcomes in type 2 diabetes: A network meta-analyses-driven approach. Diabet. Med. 2023, 40, e15157. [Google Scholar] [CrossRef]
- Tomlinson, B.; Wu, Q.Y.; Zhong, Y.M.; Li, Y.H. Advances in Dyslipidaemia Treatments: Focusing on ApoC3 and ANGPTL3 Inhibitors. J. Lipid Atheroscler. 2024, 13, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Bilson, J.; Mantovani, A.; Byrne, C.D.; Targher, G. Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 2024, 50, 101506. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fleishman, J.S.; Li, T.; Li, Y.; Ren, Z.; Chen, J.; Ding, M. Pharmacological therapy of metabolic dysfunction-associated steatotic liver disease-driven hepatocellular carcinoma. Front. Pharmacol. 2023, 14, 1336216. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, B.; Marjanovic-Haljilji, M.; Mijac, D.; Lukic, S.; Kapor, S.; Kapor, S.; Starcevic, A.; Popovic, D.; Djokovic, A. Molecular Aspects of MAFLD-New Insights on Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2023, 45, 9132–9148. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Bai, H.; Mather, B.; Hill, M.A.; Jia, G.; Sowers, J.R. Diabetic Vasculopathy: Molecular Mechanisms and Clinical Insights. Int. J. Mol. Sci. 2024, 25, 804. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial Dysfunction in Diabetes Is Aggravated by Glycated Lipoproteins; Novel Molecular Therapies. Biomedicines 2020, 9, 18. [Google Scholar] [CrossRef]
- Vellasamy, S.; Murugan, D.; Abas, R.; Alias, A.; Seng, W.Y.; Woon, C.K. Biological Activities of Paeonol in Cardiovascular Diseases: A Review. Molecules 2021, 26, 4976. [Google Scholar] [CrossRef] [PubMed]
- Sando, K.R.; Knight, M. Nonstatin therapies for management of dyslipidemia: A review. Clin. Ther. 2015, 37, 2153–2179. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Chiesa, G.; Saku, K. High-Density Lipoprotein-Targeted Therapy and Apolipoprotein A-I Mimetic Peptides. Circ. J. 2015, 79, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. 2017, 11 (Suppl. S1), S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural products in diabetes research: Quantitative literature analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Hu, M.; Zhang, D.; Yuan, S.; Li, P.; Feng, L. Medicinal and edible plants in the treatment of dyslipidemia: Advances and prospects. Chin. Med. 2022, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Mancak, M.; Altintas, D.; Balaban, Y.; Caliskan, U.K. Evidence-based herbal treatments in liver diseases. Hepatol. Forum 2024, 5, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Sima, A.V.; Stancu, C.S. Phenolic Compounds Exerting Lipid-Regulatory, Anti-Inflammatory and Epigenetic Effects as Complementary Treatments in Cardiovascular Diseases. Biomolecules 2020, 10, 641. [Google Scholar] [CrossRef]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of Berberine on Atherosclerosis. Front. Pharmacol. 2021, 12, 764175. [Google Scholar] [CrossRef]
- Huang, P. Proanthocyanidins may be potential therapeutic agents for the treatment of carotid atherosclerosis: A review. J. Int. Med. Res. 2023, 51, 3000605231167314. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Yarani, R.; Pociot, F.; Popović-Djordjević, J. Anti-diabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharmacol. Res. 2020, 155, 104723. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Choudhury, A.; Dey, B.K. An Overview on Plant Derived Phenolic Compounds and Their Role in Treatment and Management of Diabetes. J. Pharmacopunct. 2022, 25, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Nuchuchua, O.; Inpan, R.; Srinuanchai, W.; Karinchai, J.; Pitchakarn, P.; Wongnoppavich, A.; Imsumran, A. Phytosome Supplements for Delivering Gymnema inodorum Phytonutrients to Prevent Inflammation in Macrophages and Insulin Resistance in Adipocytes. Foods 2023, 12, 2257. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A. Antioxidants: Wonder drugs or quackery? Biofactors 2017, 43, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in advanced oral drug delivery system for curcumin: A systematic review. J. Control. Release 2022, 348, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Güngör Ak, A.; Akkol, E.K.; Aksu, B.; Karataş, A. Preparation and optimization of berberine phospholipid complexes using QbD approach and in vivo evaluation for anti-inflammatory, analgesic and antipyretic activity. J. Res. Pharm. 2022, 26, 370–382. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019, 14, 265–274. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef]
- Umar, H.; Wahab, H.A.; Gazzali, A.M.; Tahir, H.; Ahmad, W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers 2022, 14, 3118. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive Loaded Novel Nano-Formulations for Targeted Drug Delivery and Their Therapeutic Potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal nanomedicines: Recent advancements, challenges, opportunities and regulatory overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Deleanu, M.; Toma, L.; Sanda, G.M.; Barbălată, T.; Niculescu, L.; Sima, A.V.; Deleanu, C.; Săcărescu, L.; Suciu, A.; Alexandru, G.; et al. Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics 2023, 15, 1066. [Google Scholar] [CrossRef]
- Tripathy, S.; Patel, D.; Baro, L.; Nair, S.K. A review on phytosomes, their characterization, advancement and potential for transdermal application. J. Drug Deliv. Ther. 2013, 3, 147–152. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Morade, Y.Y.; Kothule, A.M.; Kshirsagar, S.J.; Laddha, U.D.; Salunkhe, K.S. Overview of phytosomes in treating cancer: Advancement, challenges, and future outlook. Heliyon 2023, 9, e16561. [Google Scholar] [CrossRef]
- Karpuz, M.; Silindir Gunay, M.; Ozer, Y. Liposomes and phytosomes for phytoconstituents. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Academic Press: Cambridge, MA, USA, 2020; pp. 525–553. [Google Scholar]
- Freag, M.S.; Elnaggar, Y.S.; Abdallah, O.Y. Lyophilized phytosomal nanocarriers as platforms for enhanced diosmin delivery: Optimization and ex vivo permeation. Int. J. Nanomed. 2013, 8, 2385–2397. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Narke, R. Preparation and Evaluation of Phytosome of Lawsone. Int. J. Pharm. Sci. Res. 2015, 6, 5217–5226. [Google Scholar] [CrossRef]
- Magdy, N. Phytosomes: A Novel Approach for Delivery of Herbal Constituents. J. Nutr. Diet. Probiotics 2018, 1, 180007. [Google Scholar]
- Fuior, E.V.; Mocanu, C.A.; Deleanu, M.; Voicu, G.; Anghelache, M.; Rebleanu, D.; Simionescu, M.; Calin, M. Evaluation of VCAM-1 Targeted Naringenin/Indocyanine Green-Loaded Lipid Nanoemulsions as Theranostic Nanoplatforms in Inflammation. Pharmaceutics 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Shriram, R.G.; Moin, A.; Alotaibi, H.F.; Khafagy, E.S.; Al Saqr, A.; Abu Lila, A.S.; Charyulu, R.N. Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals 2022, 15, 790. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Ali, H.; Kirby, D.J.; Mohammed, A.U.; McNeil, S.E.; Vangala, A. Environmental Scanning Electron Microscope Imaging of Vesicle Systems. Methods Mol. Biol. 2017, 1522, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Rathor, S.; Bhatt, D.C. Novel Glibenclamide–Phospholipid Complex for Diabetic Treatment: Formulation, Physicochemical Characterization, and in-vivo Evaluation. Indian J. Pharm. Educ. Res. 2022, 56, 697–705. [Google Scholar] [CrossRef]
- Altiti, A.J.; Khleifat, K.M.; Alqaraleh, M.; Shraim, A.S.; Qinna, N.; Al-Tawarah, N.M.; Al-Qaisi, T.S.; Aldmour, R.H.; Al-Tarawneh, A.; Qaralleh, H. Protective Role of Combined Crataegus Aronia Ethanol Extract and Phytosomes against Hyperglycemia and Hyperlipidemia in Streptozotocin-Induced Diabetic Rat. Biointerface Res. Appl. Chem. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, S.; Khar, R. Murraya koenigii Extract Loaded Phytosomes Prepared using Antisolvent Precipitation Technique for Improved Antidiabetic and Hypolidemic Activity. Indian J. Pharm. Educ. Res. 2022, 56, s326–s338. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Chen, Q.; He, Y.; Wang, H.; Yang, L.; Guo, S.; Meng, Z.; Cui, J.; Xue, M.; et al. Monodisperse microparticles loaded with the self-assembled berberine-phospholipid complex-based phytosomes for improving oral bioavailability and enhancing hypoglycemic efficiency. Eur. J. Pharm. Biopharm. 2016, 103, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; Leboux, R.J.T.; Glandrup, M.; van Duijn, J.; Lozano Vigario, F.; Neustrup, M.A.; Romeijn, S.; Galli, F.; Kuiper, J.; Jiskoot, W.; et al. Atomic force microscopy measurements of anionic liposomes reveal the effect of liposomal rigidity on antigen-specific regulatory T cell responses. J. Control. Release 2020, 318, 246–255. [Google Scholar] [CrossRef]
- Lee, S.H.; Sato, Y.; Hyodo, M.; Harashima, H. Size-Dependency of the Surface Ligand Density of Liposomes Prepared by Post-insertion. Biol. Pharm. Bull. 2017, 40, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef] [PubMed]
- Tutkus, M.; Akhtar, P.; Chmeliov, J.; Görföl, F.; Trinkunas, G.; Lambrev, P.H.; Valkunas, L. Fluorescence Microscopy of Single Liposomes with Incorporated Pigment-Proteins. Langmuir 2018, 34, 14410–14418. [Google Scholar] [CrossRef]
- Jain, P.; Soni, A.; Jain, P.; Bhawsar, J. Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. J. Chem. Pharm. Res. 2016, 2016, 1–6. [Google Scholar]
- Mazumder, A.; Dwivedi, A.; du Preez, J.L.; du Plessis, J. In vitro wound healing and cytotoxic effects of sinigrin-phytosome complex. Int. J. Pharm. 2016, 498, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Shulman, T.; Gibson, D.; Cohen, R.; Abra, R.; Barenholz, Y. Characterization of sterically stabilized cisplatin liposomes by nuclear magnetic resonance. Biochim. Biophys. Acta 2001, 1510, 278–291. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja Borges, C.R.L.; Silva, N.O.; Rodrigues, M.R.; Germani Marinho, M.A.; de Oliveira, F.S.; Cassiana, M.; Horn, A.P.; Parize, A.L.; Flores, D.C.; Clementin, R.M.; et al. Dimiristoylphosphatidylcholine/genistein molecular interactions: A physico-chemical approach to anti-glioma drug delivery systems. Chem. Phys. Lipids 2019, 225, 104828. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-phytosome as a potential food-grade delivery system. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- Wang, H.; Cui, Y.; Fu, Q.; Deng, B.; Li, G.; Yang, J.; Wu, T.; Xie, Y. A phospholipid complex to improve the oral bioavailability of flavonoids. Drug Dev. Ind. Pharm. 2015, 41, 1693–1703. [Google Scholar] [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Zou, J.; Zhang, X.; Wang, M.; Guo, D.; Lv, G.; Su, J.; Wang, T. The intranasal administration of Carthamus tinctorius L. extract/phospholipid complex in the treatment of cerebral infarction via the TNF-α/MAPK pathway. Biomed. Pharmacother. 2020, 130, 110563. [Google Scholar] [CrossRef] [PubMed]
- El-Menshawe, S.F.; Ali, A.A.; Rabeh, M.A.; Khalil, N.M. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: An approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018, 13, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, W.; Ye, J.; Hao, H.; Zhou, J.; Wang, R.; Liu, Y. A novel matrix dispersion based on phospholipid complex for improving oral bioavailability of baicalein: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 720–728. [Google Scholar] [CrossRef]
- Biswas, S.; Mukherjee, P.K.; Harwansh, R.K.; Bannerjee, S.; Bhattacharjee, P. Enhanced bioavailability and hepatoprotectivity of optimized ursolic acid-phospholipid complex. Drug Dev. Ind. Pharm. 2019, 45, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Gausuzzaman, S.A.L.; Saha, M.; Dip, S.J.; Alam, S.; Kumar, A.; Das, H.; Sharker, S.M.; Rashid, M.A.; Kazi, M.; Reza, H.M. A QbD Approach to Design and to Optimize the Self-Emulsifying Resveratrol-Phospholipid Complex to Enhance Drug Bioavailability through Lymphatic Transport. Polymers 2022, 14, 3220. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Petrangolini, G.; Ronchi, M.; Frattini, E.; De Combarieu, E.; Allegrini, P.; Riva, A. A New Food-grade Coenzyme Q10 Formulation Improves Bioavailability: Single and Repeated Pharmacokinetic Studies in Healthy Volunteers. Curr. Drug Deliv. 2019, 16, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhofer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, e2100613. [Google Scholar] [CrossRef]
- Petrangolini, G.; Corti, F.; Ronchi, M.; Arnoldi, L.; Allegrini, P.; Riva, A. Development of an Innovative Berberine Food-Grade Formulation with an Ameliorated Absorption: In Vitro Evidence Confirmed by Healthy Human Volunteers Pharmacokinetic Study. Evid. Based Complement. Altern. Med. 2021, 2021, 7563889. [Google Scholar] [CrossRef]
- Riva, A.; Allegrini, P.; Franceschi, F.; Togni, S.; Giacomelli, L.; Eggenhoffner, R. A novel boswellic acids delivery form (Casperome®) in the management of musculoskeletal disorders: A review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5258–5263. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef]
- Di Pierro, F.; Menghi, A.B.; Barreca, A.; Lucarelli, M.; Calandrelli, A. Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: A clinical trial. Altern. Med. Rev. 2009, 14, 154–160. [Google Scholar]
- Gilardini, L.; Pasqualinotto, L.; Di Pierro, F.; Risso, P.; Invitti, C. Effects of Greenselect Phytosome® on weight maintenance after weight loss in obese women: A randomized placebo-controlled study. BMC Complement. Altern. Med. 2016, 16, 233. [Google Scholar] [CrossRef]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Greenselect phytosome for borderline metabolic syndrome. Evid. Based Complement. Altern. Med. 2013, 2013, 869061. [Google Scholar] [CrossRef]
- Suryawanshi, J. Phytosome: An emerging trend in herbal drug treatment. J. Med. Genet. Genom. 2011, 3, 109–114. [Google Scholar]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Vigna, G.B.; Costantini, F.; Aldini, G.; Carini, M.; Catapano, A.; Schena, F.; Tangerini, A.; Zanca, R.; Bombardelli, E.; Morazzoni, P.; et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism 2003, 52, 1250–1257. [Google Scholar] [CrossRef]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Yu, Z.; Liu, X.; Chen, H.; Zhu, L. Naringenin-Loaded Dipalmitoylphosphatidylcholine Phytosome Dry Powders for Inhaled Treatment of Acute Lung Injury. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 194–204. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar]
- Sindhumol, P.G.; Thomas, M.; Mohanachandran, P.S. Phytosomes: A novel dosage form for enhancement of bioavailability of botanicals and neutraceuticals. Int. J. Pharm. Pharm. Sci. 2010, 2, 10–14. [Google Scholar]
- Riva, A.; Longo, V.; Berlanda, D.; Allegrini, P.; Masetti, G.; Botti, S.; Petrangolini, G. Healthy Protection Of Bergamot Is Linked to the Modulation of Microbiota. Appl. Microb. Res. 2020, 3, 45–51. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo-Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Bombardelli, E.; Currî, S.B.; Loggia, R.D.; Del Negro, P.; Gariboldi, P.; Tubaro, A. Anti-inflammatory activity of 18-ß-glycyrrhetinic acid in phytosome form. Fitoterapia 1989, 60, 29–37. [Google Scholar]
- Di Renzo, G. Ginkgo biloba and the central nervous system. Fitoterapia 2000, 71 (Suppl. S1), S43–S47. [Google Scholar] [CrossRef]
- Naik, S.R.; Panda, V.S. Hepatoprotective effect of Ginkgoselect Phytosome in rifampicin induced liver injury in rats: Evidence of antioxidant activity. Fitoterapia 2008, 79, 439–445. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Soltani, A.; Salmaninejad, A.; Jalili-Nik, M.; Soleimani, A.; Javid, H.; Hashemy, S.I.; Sahebkar, A. 5′-Adenosine monophosphate-activated protein kinase: A potential target for disease prevention by curcumin. J. Cell. Physiol. 2019, 234, 2241–2251. [Google Scholar] [CrossRef]
- Baradaran, S.; Hajizadeh Moghaddam, A.; Khanjani Jelodar, S.; Moradi-Kor, N. Protective Effects of Curcumin and its Nano-Phytosome on Carrageenan-Induced Inflammation in Mice Model: Behavioral and Biochemical Responses. J. Inflamm. Res. 2020, 13, 45–51. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective Effect of Ginger (Zingiber officinale Roscoe) Extract against Oxidative Stress and Mitochondrial Apoptosis Induced by Interleukin-1β in Cultured Chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Barbalata, T.; Deleanu, M.; Carnuta, M.G.; Niculescu, L.S.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Hyperlipidemia Determines Dysfunctional HDL Production and Impedes Cholesterol Efflux in the Small Intestine: Alleviation by Ginger Extract. Mol. Nutr. Food Res. 2019, 63, e1900029. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Ebrahimzadeh, A.; Mirghazanfari, S.M.; Hazrati, E.; Hadi, S.; Milajerdi, A. The effect of ginger supplementation on metabolic profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2022, 65, 102802. [Google Scholar] [CrossRef]
- Carnuta, M.G.; Deleanu, M.; Barbalata, T.; Toma, L.; Raileanu, M.; Sima, A.V.; Stancu, C.S. Zingiber officinale extract administration diminishes steroyl-CoA desaturase gene expression and activity in hyperlipidemic hamster liver by reducing the oxidative and endoplasmic reticulum stress. Phytomedicine 2018, 48, 62–69. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, Traditional Uses and Pharmacological Profile of Rose Hip: A Review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Boscaro, V.; Rivoira, M.; Sgorbini, B.; Bordano, V.; Dadone, F.; Gallicchio, M.; Pons, A.; Benetti, E.; Rosa, A.C. Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps. Pharmaceutics 2022, 14, 2371. [Google Scholar] [CrossRef]
- Adachi, Y.; Shimoyama, R. Triplet repeat primed pcr for japanese patients with myotonic dystrophy type 1. J. Neurol. Sci. 2015, 357, e235. [Google Scholar] [CrossRef]
- de Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, H.; Liu, R.; Mine, Y.; McCallum, J.; Kirby, C.; Tsao, R. Antioxidant and anti-inflammatory activities of pyranoanthocyanins and other polyphenols from staghorn sumac (Rhus hirta L.) in Caco-2 cell models. J. Funct. Foods 2016, 20, 139–147. [Google Scholar] [CrossRef]
- Dehghani, M.A.; Shakiba Maram, N.; Moghimipour, E.; Khorsandi, L.; Atefi Khah, M.; Mahdavinia, M. Protective effect of gallic acid and gallic acid-loaded Eudragit-RS 100 nanoparticles on cisplatin-induced mitochondrial dysfunction and inflammation in rat kidney. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165911. [Google Scholar] [CrossRef] [PubMed]
- Abbasalipour, H.; Hajizadeh Moghaddam, A.; Ranjbar, M. Sumac and gallic acid-loaded nanophytosomes ameliorate hippocampal oxidative stress via regulation of Nrf2/Keap1 pathway in autistic rats. J. Biochem. Mol. Toxicol. 2022, 36, e23035. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wei, Q.; Hua, W.; Liu, Y.; Liu, X.; Zhu, Y. Hepatoprotective effects of berberine on acetaminophen-induced hepatotoxicity in mice. Biomed. Pharmacother. 2018, 103, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef]
- Alam, A.; Al Arif Jahan, A.; Bari, M.S.; Khandokar, L.; Mahmud, M.H.; Junaid, M.; Chowdhury, M.S.; Khan, M.F.; Seidel, V.; Haque, M.A. Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Crit. Rev. Food Sci. Nutr. 2023, 63, 6580–6614. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, A.; Karimi, E.; Zareian, M.; Oskoueian, E. Enhancing Healthcare Outcomes and Modulating Apoptosis- and Antioxidant-Related Genes through the Nano-Phytosomal Delivery of Phenolics Extracted from Allium ampeloprasum. Genes 2023, 14, 1547. [Google Scholar] [CrossRef]
- An, J.P.; Park, E.J.; Ryu, B.; Lee, B.W.; Cho, H.M.; Doan, T.P.; Pham, H.T.T.; Oh, W.K. Oleanane Triterpenoids from the Leaves of Gymnema inodorum and Their Insulin Mimetic Activities. J. Nat. Prod. 2020, 83, 1265–1274. [Google Scholar] [CrossRef]
- Dunkhunthod, B.; Talabnin, C.; Murphy, M.; Thumanu, K.; Sittisart, P.; Eumkeb, G. Gymnema inodorum (Lour.) Decne. Extract Alleviates Oxidative Stress and Inflammatory Mediators Produced by RAW264.7 Macrophages. Oxidative Med. Cell. Longev. 2021, 2021, 8658314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, C.; Feng, W.; Li, Y.; Zhu, M.; Sun, S.; Zhang, X. Selenium-deposited tripterine phytosomes ameliorate the antiarthritic efficacy of the phytomedicine via a synergistic sensitization. Int. J. Pharm. 2020, 578, 119104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Yan, L.; Ren, Y.; Fan, J.; Zhang, X.; Zhu, S. Phytosomal tripterine with selenium modification attenuates the cytotoxicity and restrains the inflammatory evolution via inhibiting NLRP3 inflammasome activation and pyroptosis. Int. Immunopharmacol. 2022, 108, 108871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Zhou, X.; Liu, H.; Sun, H.; Ma, Z.; Wu, B. Enhancement of oral bioavailability of tripterine through lipid nanospheres: Preparation, characterization, and absorption evaluation. J. Pharm. Sci. 2014, 103, 1711–1719. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Ho, K.-H.; Cai, C.; Li, C.-W.; Yang, M.; Yi, C. Nanotechnology for diagnosis and therapy of rheumatoid arthritis: Evolution towards theranostic approaches. Chin. Chem. Lett. 2021, 32, 66–86. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, T.; Zhang, X.; Chen, J.; Sun, F.; Li, Y.; Teng, L. Oral delivery of superoxide dismutase by lipid polymer hybrid nanoparticles for the treatment of ulcerative colitis. Chin. Chem. Lett. 2022, 33, 4617–4622. [Google Scholar] [CrossRef]
- Wree, A.; Eguchi, A.; McGeough, M.D.; Pena, C.A.; Johnson, C.D.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014, 59, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Kamisli, S.; Ciftci, O.; Kaya, K.; Cetin, A.; Kamisli, O.; Ozcan, C. Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol. Ind. Health 2015, 31, 841–851. [Google Scholar] [CrossRef]
- Kalita, B.; Patwary, B.N. Formulation and in vitro Evaluation of Hesperidin-Phospholipid Complex and its Antioxidant Potential. Curr. Drug Ther. 2020, 15, 28–36. [Google Scholar] [CrossRef]
- Nuttall, S.L.; Kendall, M.J.; Bombardelli, E.; Morazzoni, P. An evaluation of the antioxidant activity of a standardized grape seed extract, Leucoselect. J. Clin. Pharm. Ther. 1998, 23, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar] [PubMed]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J.; Bai, D.C.; Liu, N.F. Proanthocyanidin from grape seeds enhances doxorubicin-induced antitumor effect and reverses drug resistance in doxorubicin-resistant K562/DOX cells. Can. J. Physiol. Pharmacol. 2005, 83, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, B.; Castillo-Henríquez, L.; Jenhani, L.; Aroua, N.; Ftouh, M.; Kalboussi, N.; Vega-Baudrit, J.; Mignet, N. Nanomedicine-based potential phyto-drug delivery systems for diabetes. J. Drug Deliv. Sci. Technol. 2023, 82, 104377. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- Miao, J.; Zang, X.; Cui, X.; Zhang, J. Autophagy, Hyperlipidemia, and Atherosclerosis. Adv. Exp. Med. Biol. 2020, 1207, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, D.T.; Michaeli, J.C.; Albers, S.; Boch, T.; Michaeli, T. Established and Emerging Lipid-Lowering Drugs for Primary and Secondary Cardiovascular Prevention. Am. J. Cardiovasc. Drugs 2023, 23, 477–495. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Ramsewak, R.S.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L.; Nitiss, J.L. Biologically active carbazole alkaloids from Murraya koenigii. J. Agric. Food Chem. 1999, 47, 444–447. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, Y.; Du, L. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J. Ethnopharmacol. 2012, 140, 345–367. [Google Scholar] [CrossRef]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Fathi, M.; Mirlohi, M.; Varshosaz, J.; Madani, G. Novel Caffeic Acid Nanocarrier: Production, Characterization, and Release Modeling. J. Nanomater. 2013, 2013, 434632. [Google Scholar] [CrossRef]
- Mangrulkar, S.; Shah, P.; Navnage, S.; Mazumdar, P.; Chaple, D. Phytophospholipid Complex of Caffeic Acid: Development, In vitro Characterization, and In Vivo Investigation of Antihyperlipidemic and Hepatoprotective Action in Rats. AAPS PharmSciTech 2021, 22, 28. [Google Scholar] [CrossRef]
- Hatamipour, M.; Jamialahmadi, T.; Ramezani, M.; Tabassi, S.A.S.; Simental-Mendía, L.E.; Sarborji, M.R.; Banach, M.; Sahebkar, A. Protective Effects of Curcumin Phytosomes against High-Fat Diet-Induced Atherosclerosis. Adv. Exp. Med. Biol. 2021, 1308, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Poruba, M.; Kazdová, L.; Oliyarnyk, O.; Malinská, H.; Matusková, Z.; Tozzi di Angelo, I.; Skop, V.; Vecera, R. Improvement bioavailability of silymarin ameliorates severe dyslipidemia associated with metabolic syndrome. Xenobiotica 2015, 45, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Patel, R.; Khambholja, K.; Patel, N. An overview of phytosomes as an advanced herbal drug delivery system. Asian J. Pharm. Sci. 2009, 4, 363–371. [Google Scholar]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 1–1419. [Google Scholar]
- Rondanelli, M.; Peroni, G.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Naso, M.; Faliva, M.A.; Tartara, A.; et al. Bergamot phytosome improved visceral fat and plasma lipid profiles in overweight and obese class I subject with mild hypercholesterolemia: A randomized placebo controlled trial. Phytother. Res. 2021, 35, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D.; Lakshmi, T. A Molecular Insight into the Role of Antioxidants in Nonalcoholic Fatty Liver Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 9233650. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Nadeau, B.; Shannon, C.; Tincopa, M. Evaluation of Dietary Approaches for the Treatment of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Nutrients 2019, 11, 3064. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Panda, V.S. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007, 27, 393–399. [Google Scholar] [CrossRef]
- Bares, J.M.; Berger, J.; Nelson, J.E.; Messner, D.J.; Schildt, S.; Standish, L.J.; Kowdley, K.V. Silybin treatment is associated with reduction in serum ferritin in patients with chronic hepatitis C. J. Clin. Gastroenterol. 2008, 42, 937–944. [Google Scholar] [CrossRef]
- Freedman, N.D.; Curto, T.M.; Morishima, C.; Seeff, L.B.; Goodman, Z.D.; Wright, E.C.; Sinha, R.; Everhart, J.E. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment. Pharmacol. Ther. 2011, 33, 127–137. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- El-Gazayerly, O.N.; Makhlouf, A.I.; Soelm, A.M.; Mohmoud, M.A. Antioxidant and hepatoprotective effects of silymarin phytosomes compared to milk thistle extract in CCl4 induced hepatotoxicity in rats. J. Microencapsul. 2014, 31, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, X.; Duan, Z.; Xu, M.; Kong, M.; Zheng, S.; Bai, L.; Chen, Y. The novel hepatoprotective mechanisms of silibinin-phospholipid complex against d-GalN/LPS-induced acute liver injury. Int. Immunopharmacol. 2023, 116, 109808. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Enhanced therapeutic potential of naringenin-phospholipid complex in rats. J. Pharm. Pharmacol. 2006, 58, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Wen, X.Y.; Kan, J.; Jin, C.H. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int. J. Biol. Macromol. 2015, 72, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Athmouni, K.; Mkadmini Hammi, K.; El Feki, A.; Ayadi, H. Development of catechin-phospholipid complex to enhance the bioavailability and modulatory potential against cadmium-induced oxidative stress in rats liver. Arch. Physiol. Biochem. 2020, 126, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Afzal, M.; Rahman, S.; Iqbal, M.; Imam, F.; Anwar, F. Antiobesity potential of ursolic acid stearoyl glucoside by inhibiting pancreatic lipase. Eur. J. Pharmacol. 2013, 709, 28–36. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front. Nutr. 2023, 10, 1163950. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Rathee, S.; Kamboj, A. Optimization and development of antidiabetic phytosomes by the Box–Behnken design. J. Liposome Res. 2018, 28, 161–172. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am. J. Chin. Med. 2014, 42, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Andrade, S.; Silva, C.; Rodrigues, I.; Guardão, L.; Guimarães, J.T.; Keating, E.; Martel, F. Chronic consumption of the dietary polyphenol chrysin attenuates metabolic disease in fructose-fed rats. Eur. J. Nutr. 2020, 59, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Imm, J.Y. The Effect of Chrysin-Loaded Phytosomes on Insulin Resistance and Blood Sugar Control in Type 2 Diabetic db/db Mice. Molecules 2020, 25, 5503. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Amjadi, S.; Shahnaz, F.; Shokouhi, B.; Azarmi, Y.; Siahi-Shadbad, M.; Ghanbarzadeh, S.; Kouhsoltani, M.; Ebrahimi, A.; Hamishehkar, H. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int. J. Pharm. 2021, 610, 121208. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef]
- Yeung, S.; Soliternik, J.; Mazzola, N. Nutritional supplements for the prevention of diabetes mellitus and its complications. J. Nutr. Intermed. Metab. 2018, 14, 16–21. [Google Scholar] [CrossRef]
- Alhabashneh, W.; Khleifat, K.; Alqaraleh, M.; Alomari, L.; Qinna, N.; Allimoun, M.; Qaralleh, H.; Farah, H.; Alqais, T. Evaluation of the Therapeutic Effect of Curcumin Phytosomes on Streptozotocin-Induced Diabetic Rats. Trop. J. Nat. Prod. Res. 2022, 6, 529–536. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Giacosa, A.; Fazia, T.; Bernardinelli, L.; Gasparri, C.; Peroni, G.; Perna, S. Berberine Phospholipid Is an Effective Insulin Sensitizer and Improves Metabolic and Hormonal Disorders in Women with Polycystic Ovary Syndrome: A One-Group Pretest-Post-Test Explanatory Study. Nutrients 2021, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Panyadee, P.; Balslev, H.; Wangpakapattanawong, P.; Inta, A. Medicinal plants in homegardens of four ethnic groups in Thailand. J. Ethnopharmacol. 2019, 239, 111927. [Google Scholar] [CrossRef] [PubMed]
- Srinuanchai, W.; Nooin, R.; Pitchakarn, P.; Karinchai, J.; Suttisansanee, U.; Chansriniyom, C.; Jarussophon, S.; Temviriyanukul, P.; Nuchuchua, O. Inhibitory effects of Gymnema inodorum (Lour.) Decne leaf extracts and its triterpene saponin on carbohydrate digestion and intestinal glucose absorption. J. Ethnopharmacol. 2021, 266, 113398. [Google Scholar] [CrossRef] [PubMed]
- Jeytawan, N.; Yadoung, S.; Jeeno, P.; Yana, P.; Sutan, K.; Naksen, W.; Wongkaew, M.; Sommano, S.R.; Hongsibsong, S. Antioxidant and Phytochemical Potential of and Phytochemicals in Gymnema inodorum (Lour.) Decne in Northern Thailand. Plants 2022, 11, 3498. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Q.; Chang, Y.; Luo, C.; Zhang, X.; Sun, S. Integrated Network Pharmacology and Cellular Assay to Explore the Mechanisms of Selenized Tripterine Phytosomes (Se@Tri-PTs) Alleviating Podocyte Injury in Diabetic Nephropathy. Curr. Pharm. Des. 2023, 29, 3073–3086. [Google Scholar] [CrossRef]
- Riva, A.; Corti, A.; Belcaro, G.; Cesarone, M.R.; Dugall, M.; Vinciguerra, G.; Feragalli, B.; Zuccarini, M.; Eggenhoffner, R.; Giacomelli, L. Interaction study between antiplatelet agents, anticoagulants, diabetic therapy and a novel delivery form of quercetin. Minerva Cardioangiol. 2019, 67, 79–83. [Google Scholar] [CrossRef]
- Di Minno, A.; Frigerio, B.; Spadarella, G.; Ravani, A.; Sansaro, D.; Amato, M.; Kitzmiller, J.P.; Pepi, M.; Tremoli, E.; Baldassarre, D. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017, 31, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Ledda, A.; Belcaro, G.; Feragalli, B.; Hosoi, M.; Cacchio, M.; Luzzi, R.; Dugall, M.; Cotellese, R. Temporary kidney dysfunction: Supplementation with Meriva® in initial, transient kidney micro-macro albuminuria. Panminerva Med. 2019, 61, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, S. A review of the chemical composition and biological activities of Callistemon lanceolatus (Sm.) Sweet. J. Appl. Pharm. Sci. 2021, 11, 065–073. [Google Scholar] [CrossRef]
- Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magaña-Rodríguez, O.R.; Lopéz-Mejía, A.; Ayala-Ruiz, L.A.; García-Calderón, A.J.; Godínez-Hernández, D.; Rios-Chavez, P. Evaluation of the toxicology, anti-lipase, and antioxidant effects of Callistemon citrinus in rats fed with a high fat-fructose diet. Pharm. Biol. 2022, 60, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Pérez, L.G.; Ayala-Ruiz, L.A.; Magaña-Rodríguez, O.R.; Piñón-Simental, J.S.; Aguilera-Méndez, A.; Godínez-Hernández, D.; Rios-Chavez, P. Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model. Pharmaceutics 2023, 15, 2178. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Tham, K.W.; Lim, A.Y.L.; Baur, L.A. The global agenda on obesity: What does this mean for Singapore? Singap. Med. J. 2023, 64, 182–187. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; El-Refaie, W.M.; El-Massik, M.A.; Abdallah, O.Y. Lecithin-based nanostructured gels for skin delivery: An update on state of art and recent applications. J. Control. Release 2014, 180, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sadeghnia, H.R.; Saberi-Karimian, M.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Effects of Curcumin on Serum Vitamin E Concentrations in Individuals with Metabolic Syndrome. Phytother. Res. 2017, 31, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; De Michele, G.; Fimiani, F.; Acerbo, V.; Scherillo, G.; Signore, G.; Rotolo, F.P.; Scialla, F.; Raucci, G.; Panico, D.; et al. Visceral adipose tissue and residual cardiovascular risk: A pathological link and new therapeutic options. Front. Cardiovasc. Med. 2023, 10, 1187735. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K. CT-based measurement of visceral adipose tissue volume as a reliable tool for assessing metabolic risk factors in prediabetes across subtypes. Sci. Rep. 2023, 13, 17902. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Raza, A.; Ullah, M.; Hendi, A.A.; Akbar, F.; Khan, S.U.; Zaman, U.; Saeed, S.; Ur Rehman, K.; Sultan, S.; et al. A Comprehensive Review: Epidemiological Strategies, Catheterization and Biomarkers used as a Bioweapon in Diagnosis and Management of Cardio Vascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101661. [Google Scholar] [CrossRef]
- Galletto, R.; Siqueira, V.L.D.; Ferreira, E.B.; de Oliveira, A.J.B.; Bazotte, R.B. Absence of antidiabetic and hypolipidemic effect of Gymnema sylvestre in non-diabetic and alloxan-diabetic rats. Braz. Arch. Biol. Technol. 2004, 47, 545. [Google Scholar] [CrossRef]
- Ohmori, R.; Iwamoto, T.; Tago, M.; Takeo, T.; Unno, T.; Itakura, H.; Kondo, K. Antioxidant activity of various teas against free radicals and LDL oxidation. Lipids 2005, 40, 849–853. [Google Scholar] [CrossRef]
- Pathan, R.A.; Bhandari, U.; Javed, S.; Nag, T.C. Anti-apoptotic potential of gymnemic acid phospholipid complex pretreatment in Wistar rats with experimental cardiomyopathy. Indian J. Exp. Biol. 2012, 50, 117–127. [Google Scholar] [PubMed]
- Naik, S.R.; Pilgaonkar, V.W.; Panda, V.S. Neuropharmacological evaluation of Ginkgo biloba phytosomes in rodents. Phytother. Res. 2006, 20, 901–905. [Google Scholar] [CrossRef] [PubMed]
- DeFeudis, F.V.; Papadopoulos, V.; Drieu, K. Ginkgo biloba extracts and cancer: A research area in its infancy. Fundam. Clin. Pharmacol. 2003, 17, 405–417. [Google Scholar] [CrossRef]
- Panda, V.S.; Naik, S.R. Cardioprotective activity of Ginkgo biloba Phytosomes in isoproterenol-induced myocardial necrosis in rats: A biochemical and histoarchitectural evaluation. Exp. Toxicol. Pathol. 2008, 60, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kishore, K.; Gupta, S.K.; Joshi, S.; Arya, D.S. Cardioprotective potential of ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 2001, 225, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Arya, D.S.; Nandave, M.; Ojha, S.K.; Kumari, S.; Joshi, S.; Mohanty, I. Myocardial salvaging effects of Ocimum sanctum in experimental model of myocardial necrosis: A haemodynamic, biochemical and histoarchitectural assessment. Curr. Sci. 2006, 91, 667–672. [Google Scholar]
- Gupta, S.K.; Prakash, J.; Srivastava, S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian. J. Exp. Biol. 2002, 40, 765–773. [Google Scholar] [PubMed]
- Uma Devi, P.; Ganasoundari, A.; Vrinda, B.; Srinivasan, K.K.; Unnikrishnan, M.K. Radiation protection by the ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat. Res. 2000, 154, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.S.; Naik, S.R. Evaluation of cardioprotective activity of Ginkgo biloba and Ocimum sanctum in rodents. Altern. Med. Rev. 2009, 14, 161–171. [Google Scholar]
- Ahmad, H.; Arya, A.; Agrawal, S.; Samuel, S.S.; Singh, S.K.; Valicherla, G.R.; Sangwan, N.; Mitra, K.; Gayen, J.R.; Paliwal, S.; et al. Phospholipid complexation of NMITLI118RT+: Way to a prudent therapeutic approach for beneficial outcomes in ischemic stroke in rats. Drug Deliv. 2016, 23, 3606–3618. [Google Scholar] [CrossRef]
- Zhang, L.L.; Tian, K.; Tang, Z.H.; Chen, X.J.; Bian, Z.X.; Wang, Y.T.; Lu, J.J. Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Cai, X.; Wu, T.; Chen, L. Protective effect of Honghua (safflower) extract on cerebral ischemia-reperfusion injury in mice. Chin. J. Tradit. Med. Sci. Technol. 2018, 25, 205–207. [Google Scholar]
- Yuan, Z.W.; Li, Y.Z.; Liu, Z.Q.; Feng, S.L.; Zhou, H.; Liu, C.X.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, H.; Li, Y.Z.; Yuan, Z.W.; Liu, C.X.; Liu, L.; Xie, Y. Baicalein Enhances the Oral Bioavailability and Hepatoprotective Effects of Silybin Through the Inhibition of Efflux Transporters BCRP and MRP2. Front. Pharmacol. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pasala, P.K.; Uppara, R.K.; Rudrapal, M.; Zothantluanga, J.H.; Umar, A.K. Silybin phytosome attenuates cerebral ischemia-reperfusion injury in rats by suppressing oxidative stress and reducing inflammatory response: In vivo and in silico approaches. J. Biochem. Mol. Toxicol. 2022, 36, e23073. [Google Scholar] [CrossRef] [PubMed]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Antimetabolic Syndrome Effect of Phytosome Containing the Combined Extracts of Mulberry and Ginger in an Animal Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 5972575. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Palachai, N.; Thukham-Mee, W.; Muchimapura, S. Memory-Enhancing Effect of a Phytosome Containing the Combined Extract of Mulberry Fruit and Ginger in an Animal Model of Ischemic Stroke with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2020, 2020, 3096826. [Google Scholar] [CrossRef]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Phytosome Loading the Combined Extract of Mulberry Fruit and Ginger Protects against Cerebral Ischemia in Metabolic Syndrome Rats. Oxidative Med. Cell. Longev. 2020, 2020, 5305437. [Google Scholar] [CrossRef]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds 2018, 30, 263–268. [Google Scholar] [PubMed]
- de Courten, B.; Jakubova, M.; de Courten, M.P.; Kukurova, I.J.; Vallova, S.; Krumpolec, P.; Valkovic, L.; Kurdiova, T.; Garzon, D.; Barbaresi, S.; et al. Effects of carnosine supplementation on glucose metabolism: Pilot clinical trial. Obesity 2016, 24, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Okyar, A.; Can, A.; Akev, N.; Baktir, G.; Sütlüpinar, N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother. Res. 2001, 15, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, B.; Dinarvand, R.; Mohammadpour, H.; Kamarul, T.; Sharifi, A.M. Dual l-Carnosine/Aloe vera Nanophytosomes with Synergistically Enhanced Protective Effects against Methylglyoxal-Induced Angiogenesis Impairment. Mol. Pharm. 2021, 18, 3302–3325. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Rajakani, R.; Gupta, V. Transcriptome-wide identification of Rauvolfia serpentina microRNAs and prediction of their potential targets. Comput. Biol. Chem. 2016, 61, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Chaudhuri, I.; Khanra, K.; Bhattacharyya, N. Biological application of green silver nanoparticle synthesized from leaf extract of Rauvolfia serpentina Benth. Asian Pac. J. Trop. Dis. 2016, 6, 549–556. [Google Scholar] [CrossRef]
- Gantait, S.; Kundu, S.; Yeasmin, L.; Ali, M.N. Impact of differential levels of sodium alginate, calcium chloride and basal media on germination frequency of genetically true artificial seeds of Rauvolfia serpentina (L.) Benth. ex Kurz. J. Appl. Res. Med. Aromat. Plants 2017, 4, 75–81. [Google Scholar] [CrossRef]
- Touqeer, S.I.; Jahan, N.; Abbas, N.; Ali, A. Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential. J. Funct. Biomater. 2022, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Raizner, A.E.; Quiñones, M.A. Coenzyme Q(10) for Patients with Cardiovascular Disease: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 609–619. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q(10): Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, A.; Veronesi, M.; Borghi, C. Noninvasive instrumental evaluation of coenzyme Q(10) phytosome on endothelial reactivity in healthy nonsmoking young volunteers: A double-blind, randomized, placebo-controlled crossover clinical trial. Biofactors 2022, 48, 1160–1165. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Techniques | References |
|---|---|---|
| Average size and shape |
| [18,38,45,46,47] [18,48,49,51,52] [18,47,48,49,50,52] [59] [52,60] [53] [54] [54] |
| Surface charge |
| [51,52,55,56] |
| Chemical composition and structure |
| [61] [38,46,52] [47,49,51,52,61,62] [63,64,65] [65] [38] [47,49,51,52,62] [47,49,52] |
| Stability |
| [38] [48,59] [62] [46,55,56] [18,47,58] |
| Encapsulation efficiency (EE%) and release behavior |
| [18,38,52,57,58] [38,46,49,52] [58] [38,46,49,52] [18] [18] [51] |
| Subject | Dose of Bioactive Compound | Cmax ± SD Unformulated Bioactive Compound | AUC ± SD Unformulated Bioactive Compound | Tmax ± SD (h) Unformulated Bioactive Compound | Cmax ± SD Bioactive Compound in Phytosome | AUC ± SD Bioactive Compound in Phytosome | Tmax ± SD Bioactive Compound in Phytosome | References |
|---|---|---|---|---|---|---|---|---|
| Sprague Dawley rats | Curcumin 340 mg/kg | 6.5 ± 4.5 (nM) | 4.8 (µg * min/mL) | 30 (min) | 33.4 ± 7.1 (nM) | 26.7 (µg * min/mL) | 15 (min) | [70] |
| Rats | Berberine 50 mg/kg | 66.01 ± 15.03 (ng/mL) | 384.45 ± 108.62 (ng * h/mL) | 0.5 (h) | 219.67 ± 6.02 (ng/mL) | 1169.19 ± 93.75 (ng * h/mL) | 2 (h) | [52] |
| Rats | Apigenin 100 mg/kg | 0.14 ± 0.15 (µg/mL) | 0.84 ± 0.42 (μg * h/mL) | 2.0 ± 0.23 (h) | 0.20 ± 0.25 (µg/mL) | 1.31 ± 0.46 (μg * h/mL) | 4.0 ± 0.34 (h) | [43] |
| Sprague Dawley rats | Baicalein 75 mg/kg | 1.61 ± 0.37 (µg/mL) | 664.68 ± 75.50 (µg * min/mL) | 170.00 ± 65.73 (min) | 8.68 ± 1.35 (µg/mL) | 1748.20 ± 280.80 (µg * min/mL) | 33.33 ± 8.67 (min) | [71] |
| Rats | Ursolic acid 20 mg/kg | 8 ± 0.21 (µg/mL) | 13.15 ± 0.34 (μg * h/mL) | 1.5 ± 1.23 (h) | 9 ± 2.17 (µg/mL) | 60.33 ± 2.19 (μg * h/mL) | 2 ± 0.43 (h) | [72] |
| Male Albino rats | Resveratrol 25 mg | 0.24 ± 0.12 (µg/mL) | 24.31 ± 4.31 (µg * min/mL) | 30 (min) | 2.27 ± 0.51 (µg/mL) | 257.15 ± 40.26 (μg * min/mL) | 60 (min) | [73] |
| Human | Curcuminoids 376 mg | 5.2 ± 0.2 (ng/mL) | 39.6 ± 1.5 (ng * h/mL) | 9.5 ± 0.2 (h) | 8.7 ± 0.4 (ng/mL) | 65.3 ± 2.3 (ng * h/mL) | 1.7 ± 0.4 (h) | [74] |
| Human | Curcuminoids 376 mg | 0.9 ± 0.1 (ng/mL) | 10.4 ± 1.3 (ng * h/mL) | 4 (h) | 18.0 ± 6.4 (ng/mL) | 86.9 ± 12.1 (ng * h/mL) | 1 (h) | [67] |
| Human | Coenzyme Q10 30 mg | 0.10 ± 0.05 (µg/mL) | 1.43 ± 1. 96 (μg * h/mL) | 16.83 ± 19.73 (h) | 0.13 ± 0.08 (µg/mL) | 3.92 ± 3.56 (µg * h/mL) | 24 ± 18.68 (h) | [75] |
| Human | Quercetin 500 mg | 10.93 ± 2.22 (ng/mL) | 4774.93 ± 1190.61 (ng * min/mL) | 290 ± 31.19 (min) | 223.10 ± 16.32 (ng/mL) | 96,163.87 ± 9291.31 (ng * min/mL) | 202.50 ± 35.97 (min) | [76] |
| Human | Curcumin 207 mg | 2.03 ± 1.79 (nM) | 19.06 ± 17.47 (nM * h) | 6.92 ± 5.96 (h) | 16.61 ± 10.10 (nM) | 147.9 ± 67.84 (nM * h) | 6.92 ± 8.34 (h) | [77] |
| Human | Berberine 188 mg | 69.95 ± 14.54 (pg/mL) | 1057 ± 117 (pg * h/mL) | 4.55 ± 0.29 (h) | 375.57 ± 41.56 (pg/mL) | 4146 ± 431 (pg * h/mL) | 4.50 ± 0.30 (h) | [78] |
| No. | Commercial Product; Doses and Administration Protocol | Company | Phytochemicals | Beneficial Effects; Mechanisms of Action | References |
|---|---|---|---|---|---|
| 1 | Berberine–phospholipid complex-based phytosomes (100 mg/kg orally administered to db/db mice 4 weeks) | - | Berberine | Anti-diabetic (decreased glucose levels in plasma and TG in the liver) | [52] |
| 2 | Casperome® phytosome (250 mg/day orally administered to subjects with musculoskeletal conditions, 1–4 weeks) | Indena | Boswellia serrata–Resin | Anti-inflammatory (lower the pain score, decrease CRP levels) | [79] |
| 3 | Curcumin phytosome (1 g × 2/day, corresponding to 200 mg curcumin, orally to humans with acute muscle injury, 4 days) | Indena | Curcuma longa L.-Rhizome | Anti-inflammatory (decreased IL-8) | [80] |
| 4 | Greenselect®/Green Tea Phytosome (2 × 150 mg/day Greenselect and 30 mg piperine orally administered to obese women, 3 months) | Indena | Camellia sinensis L.-Leaf | Bodyweight regulator (reduction in weight and fat mass, improvement of lipidic profile growth hormone, insulin-like growth factor-1, insulin and cortisol) | [81,82] |
| 5 | Monoselect Camellia (MonCam) 1 tablet eq to 300 mg/day of Greenselect phytosomes for 24 weeks | PharmExtracta (Pontenure, Italy) | Camellia sinensis | Improver of lipidic profile, antioxidant (reduced fasting glucose, increased HDL, decreased TG, reduced plasma free radicals) | [83] |
| 6 | Hawthorn Phytosome (100 mg, orally) | Indena | Flavonoids of Crataegus species | Blood pressure regulator, cardioprotective | [84,85] |
| 7 | Leucoselect®/Grape Seed Phytosome (300 mg grape procyanidin extracts eq, to healthy smoking adults, 4 weeks) | Indena | Vitis vinifera L.–Seed | Antioxidant, cardioprotective (reduced lipid peroxidation in LDL, increased the lag phase of LDL oxidation) | [86] |
| 8. | Ginseng Phytosome (100 or 200 mg per day for eight weeks orally administered to diabetic patients) | Natural Factors (Monroe, WA, USA) | Panax Ginseng | Antidiabetic effects (decreased fasting blood glucose, reduced hemoglobin A1C values for 200 mg dose) | [18,87] |
| 9 | Naringenin Phytosome (Approx 3 mg Naringenin eq, intratracheary administered to rats with acute lung injury, 4 h) | - | Citrus aurantium | Anti-inflammatory, antioxidant (increased SOD2 mRNA, decreased COX2 and ICAM-1 gene expression, decreased p-p38MAPK in the lungs) | [88] |
| 10 | Quercefit™ Phytosome (1–2 tabs/day administered to human subjects with both asthma and rhinitis, for 30 days) | Indena | Quercetin | Antioxidant (reduction of plasma-free radicals) | [89] |
| 11 | Siliphos® (120 mg, orally administered) | Indena | Silybin of Silybum marianum | Antioxidant, hepatoprotective | [85,90] |
| 12 | Green Tea Phytosome 1 capsule 2–3 times/day orally administered to obese subjects for 90 days) | Natural Factors | Green tea polyphenols | Antioxidant, bodyweight regulator (thermogenic effect, improvements in weight and body mass index) | [91] |
| 13 | Ubiqsome™ Phytosome (150–300 mg corresponding to 30–60 mg CoQ10, administered orally to healthy humans for two periods of two weeks) | Indena | Co-enzyme Q10 | Antioxidant; a good absorbance of CoQ10 | [75] |
| 14 | Vazguard™ Phytosome (1000 mg/L of bergamot phytosome subjected to simulated gastric digestion and further incubated to fecal slurries from healthy women) | Indena | Bergamot extract | Regulator of plasma glucose and lipid levels, bodyweight regulator (modulator of gut microbiota: Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria) | [92] |
| 15 | Vazguard™/Naringin Phytosome (oral administration to type 2 diabetic patients) | Indena | Bergamot extract | Antioxidant, cardioprotective (improvement in lipid profile, reduction in small, dense LDLs and decreased glucose levels in plasma) | [93] |
| Study Type | Disorder | Bioactive Compounds Formulated in Phytosomes; Beneficial Effects |
|---|---|---|
| Preclinical | Oxidative and Inflammatory stress |
|
| Dyslipidemia |
| |
| Hepatic disorders |
| |
| Diabetes mellitus |
| |
| Metabolic syndrome | ||
| Cardiovascular diseases |
| |
| Clinical | Oxidative and Inflammatory stress | |
| Dyslipidemia |
| |
| Hepatic disorders |
| |
| Diabetes mellitus | ||
| Metabolic syndrome |
| |
| Cardiovascular diseases |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, L.; Deleanu, M.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Stancu, C.S. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. Int. J. Mol. Sci. 2024, 25, 4162. https://doi.org/10.3390/ijms25084162
Toma L, Deleanu M, Sanda GM, Barbălată T, Niculescu LŞ, Sima AV, Stancu CS. Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. International Journal of Molecular Sciences. 2024; 25(8):4162. https://doi.org/10.3390/ijms25084162
Chicago/Turabian StyleToma, Laura, Mariana Deleanu, Gabriela Maria Sanda, Teodora Barbălată, Loredan Ştefan Niculescu, Anca Volumnia Sima, and Camelia Sorina Stancu. 2024. "Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders" International Journal of Molecular Sciences 25, no. 8: 4162. https://doi.org/10.3390/ijms25084162
APA StyleToma, L., Deleanu, M., Sanda, G. M., Barbălată, T., Niculescu, L. Ş., Sima, A. V., & Stancu, C. S. (2024). Bioactive Compounds Formulated in Phytosomes Administered as Complementary Therapy for Metabolic Disorders. International Journal of Molecular Sciences, 25(8), 4162. https://doi.org/10.3390/ijms25084162