Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin—A Comprehensive Review
Abstract
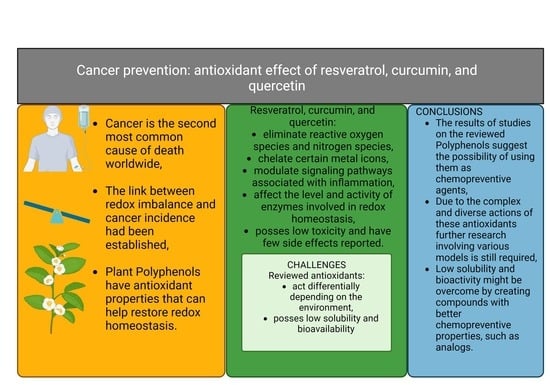
:1. Introduction
2. Oxygen, Oxidative and Nitrosative Stress
3. Sources of Reactive Oxygen and Nitrogen Species
4. Carcinogenesis
5. Reactive Oxygen Species in Carcinogenesis

6. Nitrogen Species in Carcinogenesis
7. Chemoprevention
Role of Antioxidants in Chemoprevention
8. Resveratrol
8.1. Effect of Resveratrol on Metabolism and Detoxification of Carcinogens
8.2. Resveratrol Eliminates ROS and Prevents DNA Synthesis and Its Oxidative Damage
8.3. Resveratrol and Regulation of Nitric Oxide Synthesis
8.4. Effect of Resveratrol on Lipid Metabolism
8.5. Effect of Antioxidant Properties of Resveratrol on Cyclooxygenases
8.6. Interaction between Resveratrol and Nrf2
8.7. Link between Resveratrol and Tumor Necrosis Factor
8.8. Problems Related to the Use of Resveratrol
| Research Model | Carcinogenic Factor | Effect Exerted by Resveratrol | Target of Chemoprevention | Author |
|---|---|---|---|---|
| In vivo | Benzo[a]pyrene | Suppression of CYP1A1 expression, inhibition of DNA adduct formation | Lung cancer | [76] |
| In vitro | TCDD | Inhibition of CYP1B1 activity and expression, prevention of DNA damage | Breast cancer | [83] |
| In vitro | tBHP, Fe2+ | Alleviation of oxidative stress | Pheochromocytoma | [84] |
| In vivo | DENA | Alleviation of oxidative | Liver cancer | [85] |
| stress | ||||
| In vitro | – | Elimination of the tyrosyl radical | Leukemia | [88] |
| In vitro | – | Modulation of iNOS and reduction of NO synthesis | Gastric cancer | [89] |
| In vitro | LPS | Downregulation of iNOS expression and inhibition of NO synthesis | Colon cancer | [90] |
| In vitro | UVB | Reduction of oxidative stress, inhibition of NF-κb | Skin cancer | [110] |
| In vitro | TNF | Reduction of ROS production and lipid peroxidation, suppression of AP-1 and NF-κb | Lymphoma, cervical cancer, glioma, leukemia | [103] |
9. Curcumin
9.1. Antioxidant Properties of Curcumin
9.2. The Role of Curcumin in the Detoxification of Carcinogens
9.3. Curcumin Counteracts the Oxidation of Lipids, Proteins, and DNA
9.4. Curcumin Has Inhibitory Effects on NF-κB, AP-1, and Related Molecules
9.5. Curcumin Increases Heme Oxygenase Expression and Activity
9.6. The Effect of Curcumin in Different Research Models and Problems Associated with Its Use as a Chemopreventive Agent
10. Quercetin
10.1. Antioxidant Properties of Quercetin
10.2. Effects of Quercetin on Carcinogen Metabolism
10.3. Quercetin Alleviates Oxidative Damage to DNA
10.4. Elimination of Reactive Oxygen and Nitrogen Species by Quercetin
10.5. Effect of Quercetin on Nitric Oxide Synthesis
10.6. Quercetin Counteracts Lipid Peroxidation and LDL Oxidation
10.7. Effect of Quercetin on COX-2, NF-κB, and Nrf2
10.8. Quercetin in Chemoprevention and Problems Related to the Use of Quercetin
11. Resveratrol, Curcumin, and Quercetin Targeting the Thioredoxin System
12. Conclusions
Funding
Conflicts of Interest
References
- Weiss, C. One in Four Dies of Cancer. Questions About the Epidemiology of Malignant Tumours. Recent Results Cancer Res. 2021, 218, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Dawane, J.S.; Pandit, V.A. Understanding Redox Homeostasis and Its Role in Cancer. J. Clin. Diagn. Res. 2012, 6, 1796. [Google Scholar] [CrossRef] [PubMed]
- Ługowski, M.; Saczko, J.; Kulbacka, J.; Banaś, T. Reactive oxygen and nitrogen species. Pol. Merkur. Lek. 2011, 31, 313–317. [Google Scholar] [PubMed]
- Szymańska, B.; Kawecki, M.; Knefel, G. Clinical aspects of hyperbaric oxygenation. Wiad. Lek. 2006, 59, 105–109. [Google Scholar] [PubMed]
- Kalisz, O.; Wolski, T.; Gerkowicz, M.; Smorawski, M. Reactive oxygen species [RTF] and their role in the pathogenesis of some diseases. Ann. Univ. Mariae Curie-Skłodowska. Sect. DD Med. Vet. 2007, 62, 87–99. [Google Scholar]
- Puzanowska-Tarasiewicz, H.; Starczewska, B.; Kuźmicka, L. Reactive oxygen specis. Bromatol. Chem. Toksykol. 2008, 41, 1007–1015. [Google Scholar]
- Krukiewicz, K. The phenomenon of singlet oxygen. CHEMIK Nauka-Tech. Rynek 2011, 65, 1190–1192. [Google Scholar]
- Hrycay, E.G.; Bandiera, S.M. Involvement of Cytochrome P450 in Reactive Oxygen Species Formation and Cancer. Adv. Pharmacol. 2015, 74, 35–84. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Saczko, J.; Chwiłkowska, A. Oxidative stress in cells damage processes. Pol. Merkur. Lek. 2009, 27, 44–47. [Google Scholar]
- Nawrot, R.; Goździcka-Józefiak, A. Biotechnology to combat reactive oxygen species. Comm. Biotechnol. PAS 2002, 57, 88–101. [Google Scholar]
- Igielska-Kalwat, J.; Gościańska, J.; Nowak, I. Carotenoids as natural antioxidants. Postep. Hig. Med. Dosw. Online 2015, 69, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Gałecka, E.; Mrowicka, M.; Malinowska, K.; Gałecki, P. Role of free radicals in the physiological processes. Pol. Merkur. Lek. 2008, 24, 446–448. [Google Scholar] [PubMed]
- Sokołowska, M.; Włodek, L. The good and bad sides of nitric oxide. Cardiol. J. 2001, 8, 467–474. [Google Scholar]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. Med. Dosw. Online 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Faraci, F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol. Med. 2008, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Zapora, E.; Jarocka, I. Hemoglobin–source of reactive oxygen species. Postep. Hig. Med. Dosw. Online 2013, 67, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, P.; Wiktorowicz, K.; Goździcka-Józefiak, A.; Parucki, R.; Wysocki, H. Disturbances in mitochondrial biosynthesis of acetyl-CoA and their role in the prevention of ischemic heart disease. Kardiol. Pol. 2008, 66, 1215–1220. [Google Scholar] [PubMed]
- Potargowicz, E.; Szerszenowicz, E.; Staniszewska, M.; Nowak, D. Mitochondria as an source of reactive oxygen species. Postepy Hig. Med. Dosw. 2005, 59, 259–266. [Google Scholar]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Reakcja Fentona. Kosmos 2014, 63, 309–314. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Łagowska-Lenard, M.; Bielewicz, J.; Raszewski, G.; Stelmasiak, Z.; Bartosik-Psujek, H. Oxidative stress in cerebral stroke. Pol. Merkur. Lek. Lek. 2008, 25, 205–208. [Google Scholar]
- Couch, D.B. Carcinogenesis: Basic Principles. Drug Chem. Toxicol. 1996, 19, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Domagała, W. Molecular basis of carcinogenesis and signaling pathways of some tumors of the central nervous system. Pol. Przegląd Neurol. 2007, 3, 127–141. [Google Scholar]
- Sagar, J.; Chaib, B.; Sales, K.; Winslet, M.; Seifalian, A. Role of stem cells in cancer therapy and cancer stem cells: A review. Cancer Cell Int. 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- De Civetta, M.T.M.; Civetta, J.D. Carcinogenesis. Salud Publica Mex. 2011, 53, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer Chemoprevention Through Dietary Antioxidants: Progress and Promise. Antioxid. Redox Signal. 2008, 10, 475–510. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2009, 38, 96–109. [Google Scholar] [CrossRef]
- Rutkowski, R.; Pancewicz, S.A.; Rutkowski, K.; Rutkowska, J. Reactive oxygen and nitrogen species in inflammatory process. Pol. Merkur. Lek. 2007, 23, 131–136. [Google Scholar]
- Weyemi, U.; Redon, C.E.; Parekh, P.R.; Dupuy, C.; Bonner, W.M. NADPH Oxidases NOXs and DUOXs as Putative Targets for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2013, 13, 502–514. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004, 423, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Tatemichi, M.; Sawa, T. Chemical basis of inflammation-induced carcinogenesis. Arch. Biochem. Biophys. 2003, 417, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Nair, J. Accumulation of lipid peroxidation-derived DNA lesions: Potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat. Res. Mol. Mech. Mutagen. 2005, 591, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhou, X.; Yu, H.; Dong, M.; Taghizadeh, K.; Wishnok, J.S.; Tannenbaum, S.R.; Dedon, P.C. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinog. 2007, 28, 1807–1813. [Google Scholar] [CrossRef]
- Almeida, K.H.; Sobol, R.W. A unified view of base excision repair: Lesion-dependent protein complexes regulated by post-translational modification. DNA Repair 2007, 6, 695–711. [Google Scholar] [CrossRef]
- Engelward, B.P.; Weeda, G.; Wyatt, M.D.; Broekhof, J.L.M.; de Wit, J.; Donker, I.; Allan, J.M.; Gold, B.; Hoeijmakers, J.H.J.; Samson, L.D. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc. Natl. Acad. Sci. USA 1997, 94, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Hang, B.; Singer, B.; Margison, G.P.; Elder, R.H. Targeted deletion of alkylpurine-DNA-N-glycosylase in mice eliminates repair of 1, N 6 -ethenoadenine and hypoxanthine but not of 3, N 4 -ethenocytosine or 8-oxoguanine. Proc. Natl. Acad. Sci. USA 1997, 94, 12869–12874. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, T.M.; Dong, L.; Connor, E.E.; Meira, L.B.; Samson, L.D.; Wyatt, M.D.; Cao, W. Oxanine DNA Glycosylase Activity from Mammalian Alkyladenine Glycosylase. J. Biol. Chem. 2004, 279, 38177–38183. [Google Scholar] [CrossRef] [PubMed]
- Saparbaev, M.; Langouët, S.; Privezentzev, C.V.; Guengerich, F.P.; Cai, H.; Elder, R.H.; Laval, J. 1,N 2-Ethenoguanine, a Mutagenic DNA Adduct, Is a Primary Substrate of Escherichia coliMismatch-specific Uracil-DNA Glycosylase and Human Alkylpurine-DNA-N-Glycosylase. J. Biol. Chem. 2002, 277, 26987–26993. [Google Scholar] [CrossRef] [PubMed]
- Wuenschell, G.E.; O’Connor, T.R.; Termini, J. Stability, Miscoding Potential, and Repair of 2‘-Deoxyxanthosine in DNA: Implications for Nitric Oxide-Induced Mutagenesis. Biochemistry 2003, 42, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Ringvoll, J.; Moen, M.N.; Nordstrand, L.M.; Meira, L.B.; Pang, B.; Bekkelund, A.; Dedon, P.C.; Bjelland, S.; Samson, L.D.; Falnes, P.; et al. AlkB Homologue 2–Mediated Repair of Ethenoadenine Lesions in Mammalian DNA. Cancer Res 2008, 68, 4142–4149. [Google Scholar] [CrossRef] [PubMed]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.-W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.; Nair, J.; Winde, G.; Velic, I.; Bartsch, H. Increased levels of promutagenic etheno-dna adducts in colonic polyps of FAP patients. Int. J. Cancer 2000, 87, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. Regulated Protein Denitrosylation by Cytosolic and Mitochondrial Thioredoxins. Science 2008, 320, 1050–1054. [Google Scholar] [CrossRef]
- Iyer, A.K.V.; Azad, N.; Wang, L.; Rojanasakul, Y. Role of S-nitrosylation in apoptosis resistance and carcinogenesis. Nitric Oxide 2008, 19, 146–151. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free. Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; LaRusso, N.F.; Nishioka, N.; Nakabeppu, Y.; Gores, G.J. Human Ogg1, a protein involved in the repair of 8-oxoguanine, is inhibited by nitric oxide. Cancer Res. 2001, 61, 6388–6393. [Google Scholar] [PubMed]
- Qu, J.; Liu, G.-H.; Huang, B.; Chen, C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of Cysteines 93 and 310. Nucleic Acids Res. 2007, 35, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.E.; Ying, L.; Hofseth, A.B.; Jelezcova, E.; Sobol, R.W.; Ambs, S.; Harris, C.C.; Espey, M.G.; Hofseth, L.J.; Wyatt, M.D. Differential effects of reactive nitrogen species on DNA base excision repair initiated by the alkyladenine DNA glycosylase. Carcinogenesis 2009, 30, 2123–2129. [Google Scholar] [CrossRef]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Mojic, M.; Mijatovic, S.; Maksimovic-Ivanic, D.; Dinic, S.; Grdovic, N.; Miljkovic, D.; Stosic-Grujicic, S.; Tumino, S.; Fagone, P.; Mangano, K.; et al. Saquinavir-NO-targeted S6 protein mediates sensitivity of androgen-dependent prostate cancer cells to TRAIL. Cell Cycle 2012, 11, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Baer-Dubowska, W. Chemoprevention—Prevention and supported therapy of head and neck cancer. Adv. Head Neck Surg. 2003, 2, 3–14. [Google Scholar]
- Su, Z.-Y.; Shu, L.; Khor, T.O.; Lee, J.H.; Fuentes, F.; Kong, A.-N.T. A Perspective on Dietary Phytochemicals and Cancer Chemoprevention: Oxidative Stress, Nrf2, and Epigenomics. Top Curr. Chem. 2013, 329, 133. [Google Scholar] [CrossRef]
- Maliszewska, M. Curcumin, indole-3-carbinol and resveratrol in chemoprevention of breast cancer. Postępy Fitoter. 2012. [Google Scholar]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a Chemopreventive Agent: A Promising Molecule for Fighting Cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Kelkel, M.; Jacob, C.; Dicato, M.; Diederich, M. Potential of the Dietary Antioxidants Resveratrol and Curcumin in Prevention and Treatment of Hematologic Malignancies. Molecules 2010, 15, 7035–7074. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxidative Med. Cell. Longev. 2015, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pieszka, M.; Szczurek, P.; Ropka-Molik, K.; Oczkowicz, M.; Pieszka, M. The role of resveratrol in the regulation of cell metabolism—A review. Postepy Hig. Med. Dosw. 2016, 70, 117–123. [Google Scholar] [CrossRef]
- Kumar, S.; Chang, Y.-C.; Lai, K.-H.; Hwang, T.-L. Resveratrol, a Molecule with Anti-Inflammatory and Anti-Cancer Activities: Natural Product to Chemical Synthesis. Curr. Med. Chem. 2021, 28, 3773–3786. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef]
- Boue, S.M.; Cleveland, T.E.; Carter-Wientjes, C.; Shih, B.Y.; Bhatnagar, D.; McLachlan, J.M.; Burow, M.E. Phytoalexin-Enriched Functional Foods. J. Agric. Food Chem. 2009, 57, 2614–2622. [Google Scholar] [CrossRef]
- Song, P.; Yu, X.; Yang, W.; Wang, Q. Natural phytoalexin stilbene compound resveratrol and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. Sci. Rep. 2021, 11, 16509. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative Analysis of the Antitumor Activity of Cis- and Trans-Resveratrol in Human Cancer Cells with Different p53 Status. Molecules 2021, 26, 5586. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Kuczmarska, A.; Ksiazek, K. Biological multifunctionality of resveratrol and its derivatives. Postepy Biochem. 2015, 61, 336–343. [Google Scholar] [PubMed]
- Mikstacka, R.; Dutkiewicz, Z. New Perspectives of CYP1B1 Inhibitors in the Light of Molecular Studies. Processes 2021, 9, 817. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Rogers, I.; Han, R.; Savouret, J.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 2003, 23, 255–261. [Google Scholar] [CrossRef]
- Kisková, T.; Kassayová, M. Resveratrol Action on Lipid Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 2704. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Hurh, Y.-J.; Na, H.-K.; Kim, J.-H.; Chun, Y.-J.; Kim, D.-H.; Kang, K.-S.; Cho, M.-H.; Surh, Y.-J. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis 2004, 25, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Almatroodi, S.A.; Alsahli, M.A.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Resveratrol, a Plant Polyphenol, and Its Role in the Therapy of Various Types of Cancer. Molecules 2022, 27, 2665. [Google Scholar] [CrossRef]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Mol. Mech. Mutagen. 2008, 658, 68–94. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.; Pezzuto, J.M. Cancer Chemopreventive Activity of Resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, C.; Ye, J.L.; Lv, Y.; Wang, L.; Chen, Z.; Jiang, Z.Y. Protection of Porcine Intestinal-Epithelial Cells from Deoxynivalenol-Induced Damage by Resveratrol via the Nrf2 Signaling Pathway. J. Agric. Food Chem. 2018, 67, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Chanvitayapongs, S.; Draczynska-Lusiak, B.; Sun, A.Y. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. NeuroReport 1997, 8, 1499–1502. [Google Scholar] [CrossRef]
- Bishayee, A.; Petit, D.M.; Samtani, K. Angioprevention is Implicated in Resveratrol Chemoprevention of Experimental Hepatocarcinogenesis. J. Carcinog. Mutagen. 2010, 1, 102. [Google Scholar] [CrossRef]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 496, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, M.; Lepoivre, M.; Elleingand, E.; Gerez, C.; Guittet, O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998, 421, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Holian, O.; Wahid, S.; Atten, M.J.; Attar, B.M. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Am. J. Physiol. Liver Physiol. 2002, 282, G809–G816. [Google Scholar] [CrossRef]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef]
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945. [Google Scholar] [CrossRef] [PubMed]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of OxLDL in cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Janarthan, M.; Ramachandran, H.K.; Chatterjee, M. Role of 5-lipoxygenase in resveratrol mediated suppression of 7,12-dimethylbenz(α)anthracene-induced mammary carcinogenesis in rats. Eur. J. Pharmacol. 2011, 668, 99–106. [Google Scholar] [CrossRef]
- Martinez, J.; Moreno, J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000, 59, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Kolasińska-Bzoma, M.; Sitarz, R.; Skórzewska, M.; Polkowski, W.; Maciejewski, R. Cyclooxygenase-2 and Its Role in Carcinogenesis; Zeszyty Naukowe Towarzystwa Doktorantów Uniwersytetu Jagiellońskiego, Nauki Ścisłe: Krakow, Poland, 2012; Volume 5. [Google Scholar]
- Feng, M.; Zhong, L.-X.; Zhan, Z.-Y.; Huang, Z.-H.; Xiong, J.-P. Resveratrol Treatment Inhibits Proliferation of and Induces Apoptosis in Human Colon Cancer Cells. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jia, B.; Song, X.; Kong, Q.-Y.; Wu, M.-L.; Qiu, Z.-W.; Li, H.; Liu, J. Preventive Potential of Resveratrol in Carcinogen-Induced Rat Thyroid Tumorigenesis. Nutrients 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2009, 127, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Patrycja, P.-T.; Agata, S.; Jeremi, W.; Ruslan, Z.; Magdalena, S.-K. The Role of Nrf2 Factor and its Activators in the Pathogenesis of Selected Nervous System Diseases. Postępy Biol. Komórki 2020, 47, 101–118. [Google Scholar]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage:: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Kang, H.J.; Bin Hong, Y.; Kim, H.J.; Wang, A.; Bae, I. Bioactive food components prevent carcinogenic stress via Nrf2 activation in BRCA1 deficient breast epithelial cells. Toxicol. Lett. 2012, 209, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-κB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Han, Y.; Jo, H.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol as a Tumor-Suppressive Nutraceutical Modulating Tumor Microenvironment and Malignant Behaviors of Cancer. Int. J. Mol. Sci. 2019, 20, 925. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- da Costa, D.C.F.; Rangel, L.P.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Feng, M.; Zhong, L.-X.; Zhan, Z.-Y.; Huang, Z.-H.; Xiong, J.-P. Enhanced antitumor efficacy of resveratrol-loaded nanocapsules in colon cancer cells: Physicochemical and biological characterization. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 375–382. [Google Scholar] [PubMed]
- Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Parekh, H.S.; Popat, A. Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J. Colloid Interface Sci. 2016, 462, 368–374. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-κB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Szczepański, M.; Grzanka, A. Chemopreventive and anticancer properties of curcumin. Nowotwory. J. Oncol. 2009, 59, 377. [Google Scholar]
- Padhye, S.; Chavan, D.; Pandey, S.; Deshpande, J.; Swamy, K.; Sarkar, F. Perspectives on Chemopreventive and Therapeutic Potential of Curcumin Analogs in Medicinal Chemistry. Mini-Rev. Med. Chem. 2010, 10, 372–387. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes–A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Biswas, S.K.; McClure, D.; Jimenez, L.A.; Megson, I.L.; Rahman, I. Curcumin Induces Glutathione Biosynthesis and Inhibits NF-κB Activation and Interleukin-8 Release in Alveolar Epithelial Cells: Mechanism of Free Radical Scavenging Activity. Antioxid. Redox Signal. 2005, 7, 32–41. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, H.; Sun, X.; Wang, X.; Han, M.; Lu, Z.; Cheng, P.; Hussain, M.A.; Zhang, X. Dual Role of Dietary Curcumin Through Attenuating AFB1-Induced Oxidative Stress and Liver Injury via Modulating Liver Phase-I and Phase-II Enzymes Involved in AFB1 Bioactivation and Detoxification. Front. Pharmacol. 2018, 9, 554. [Google Scholar] [CrossRef]
- Thapliyal, R.; Maru, G. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem. Toxicol. 2001, 39, 541–547. [Google Scholar] [CrossRef]
- Choi, H.; Chun, Y.; Shin, Y.J.; Ye, S.K.; Kim, M.; Park, J. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008, 99, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary Supplementation of Curcumin Enhances Antioxidant and Phase II Metabolizing Enzymes in ddY Male Mice: Possible Role in Protection against Chemical Carcinogenesis and Toxicity. Basic Clin. Pharmacol. Toxicol. 2003, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Vinayak, M. Long Term Effect of Curcumin in Restoration of Tumour Suppressor p53 and Phase-II Antioxidant Enzymes via Activation of Nrf2 Signalling and Modulation of Inflammation in Prevention of Cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A.; Thippeswamy, N. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol. Cell. Biochem. 2002, 229, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Y.; Chen, W.-F.; Zhou, B.; Yang, L.; Liu, Z.-L. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 70–77. [Google Scholar] [CrossRef]
- Okada, K.; Wangpoengtrakul, C.; Tanaka, T.; Toyokuni, S.; Uchida, K.; Osawa, T. Curcumin and Especially Tetrahydrocurcumin Ameliorate Oxidative Stress-Induced Renal Injury in Mice. J. Nutr. 2001, 131, 2090–2095. [Google Scholar] [CrossRef]
- Nisari, M.; Yılmaz, S.; Ertekin, T.; Ceylan, D.; İnanç, N.; Al, O.; Ülger, H. Effects of Curcumin on Lipid Peroxidation and Antioxidant Enzymes in Kidney, Liver, Brain and Testis of Mice Bearing Ehrlich Solid Tumor. Proceedings 2017, 1, 994. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Biswas, J.; Sinha, D.; Mukherjee, S.; Roy, S.; Siddiqi, M.; Roy, M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum. Exp. Toxicol. 2010, 29, 513–524. [Google Scholar] [CrossRef]
- Hosseinimehr, S.; Shafaghati, N.; Hedayati, M. Protective effects of curcumin against genotoxicity induced by 131-iodine in human cultured lymphocyte cells. Pharmacogn. Mag. 2014, 10, 106–110. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.-N. Curcumin: A therapeutic strategy in cancers by inhibiting the canonical WNT/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 323. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aggarwal, B.B. Activation of Transcription Factor NF-κB Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. The Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Young, M.R.I.; Colburn, N.H. The role of AP-1, NF-κB and ROS/NOS in skin carcinogenesis: The JB6 model is predictive. Mol. Cell Biochem. 2004, 234–235, 185–193. [Google Scholar]
- Prakobwong, S.; Khoontawad, J.; Yongvanit, P.; Pairojkul, C.; Hiraku, Y.; Sithithaworn, P.; Pinlaor, P.; Aggarwal, B.B.; Pinlaor, S. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int. J. Cancer 2010, 129, 88–100. [Google Scholar] [CrossRef]
- Pinlaor, S.; Yongvanit, P.; Prakobwong, S.; Kaewsamut, B.; Khoontawad, J.; Pinlaor, P.; Hiraku, Y. Curcumin reduces oxidative and nitrative DNA damage through balancing of oxidant–antioxidant status in hamsters infected with Opisthorchis viverrini. Mol. Nutr. Food Res. 2009, 53, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Chu, L.; Hua, K.; Chao, L.K. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J. Cell. Physiol. 2008, 215, 603–612. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Huang, Y.; Kong, A.-N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Sahin, N.; Tastan, H.; Özercan, I.H.; Güler, O.; Kahraman, N.; Kucuk, O.; Ozpolat, B. Chemopreventive and Antitumor Efficacy of Curcumin in a Spontaneously Developing Hen Ovarian Cancer Model. Cancer Prev. Res. 2018, 11, 59–67. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, Q.Y.; Li, H.Y.; Zhou, X.; Liu, Y.; Zhang, H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol. Biochem. Behav. 2014, 126, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2017, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Ipar, V.S.; Dsouza, A.; Devarajan, P.V. Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 459–480. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Yamakoshi, H.; Sato, A.; Ohori, H.; Kakudo, Y.; Kudo, C.; Takahashi, Y.; Watanabe, M.; Takano, H.; Ishioka, C.; et al. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci. 2009, 100, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Al-Hujaily, E.M.; Mohamed, A.G.; Al-Sharif, I.; Youssef, K.M.; Manogaran, P.S.; Al-Otaibi, B.; Al-Haza’a, A.; Al-Jammaz, I.; Al-Hussein, K.; Aboussekhra, A. PAC, a novel curcumin analogue, has anti-breast cancer properties with higher efficiency on ER-negative cells. Breast Cancer Res. Treat. 2010, 128, 97–107. [Google Scholar] [CrossRef]
- Kałwa, K. Antioxidant Properties of Flavonoids and Their Impact on Human Health. Kosmos 2019, 68, 153–159. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytotherapy Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Pałys, A.; Budzisz, E. Quercetin—Structure, function and clinical usage. Farm. Pol. 2011, 67, 18–23. [Google Scholar]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.-J.; Frei, B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016, 9, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. Vitr. 2006, 20, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, T.; Kim, G.-H. Quercetin acts as an antioxidant and downregulates CYP1A1 and CYP1B1 against DMBA-induced oxidative stress in mice. Oncol. Rep. 2012, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jee, S.-C.; Kim, K.-S.; Kim, H.-S.; Yu, K.-N.; Sung, J.-S. Quercetin and Isorhamnetin Attenuate Benzo[a]pyrene-Induced Toxicity by Modulating Detoxification Enzymes through the AhR and NRF2 Signaling Pathways. Antioxidants 2021, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.K.; Khanduja, K.L. Impact of Quercetin Consumption on Phase-I and Phase-II Drug-Metabolizing Enzymes in Mice. J. Clin. Biochem. Nutr. 1993, 14, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.; Kepa, J.; Pickwell, G.; Quattrochi, L. Induction of human NAD(P)H:quinone oxidoreductase (NQO1) gene expression by the flavonol quercetin. Toxicol. Lett. 2001, 119, 49–57. [Google Scholar] [CrossRef]
- Darband, S.G.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020, 253, 117584. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Seufi, A.M.; Ibrahim, S.S.; Elmaghraby, T.K.; E Hafez, E. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: Molecular and histological evidences. J. Exp. Clin. Cancer Res. 2009, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Ebeler, S.E. Quercetin inhibits hydrogen peroxide-induced DNA damage and enhances DNA repair in Caco-2 cells. Food Chem. Toxicol. 2009, 47, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hou, Q.; Lei, J.; Wolf, P.G.; Ayansola, H.; Zhang, B. Quercetin Alleviates Intestinal Oxidative Damage Induced by H2O2 via Modulation of GSH: In Vitro Screening and In Vivo Evaluation in a Colitis Model of Mice. ACS Omega 2020, 5, 8334–8346. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.E.; Burd, R. Quercetin as a Systemic Chemopreventative Agent: Structural and Functional Mechanisms. Mini-Reviews Med. Chem. 2011, 11, 1216–1221. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2016, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, R.V.; Nagini, S. Quercetin suppresses cytochrome P450 mediated ROS generation and NFκB activation to inhibit the development of 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinomas. Free. Radic. Res. 2011, 46, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): Key role of quercetin in cancer prevention. Tumor Biol. 2015, 36, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Mallaiah, S.H.; Patil, R.K. Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef]
- Zhang, M. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced release of nitric oxide. Chem. Interact. 2001, 137, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Konwar, B.K. Molecular docking studies of quercetin and its analogues against human inducible nitric oxide synthase. SpringerPlus 2012, 1, 69. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Choi, Y.H.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int. Immunopharmacol. 2013, 17, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Huang, Y.-T.; Tsai, S.-H.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinog. 1999, 20, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Košinová, P.; Berka, K.; Wykes, M.; Otyepka, M.; Trouillas, P. Positioning of Antioxidant Quercetin and Its Metabolites in Lipid Bilayer Membranes: Implication for Their Lipid-Peroxidation Inhibition. J. Phys. Chem. B 2012, 116, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive Effect of Quercetin in MNU and Testosterone Induced Prostate Cancer of Sprague-Dawley Rats. Nutr. Cancer 2013, 66, 38–46. [Google Scholar] [CrossRef]
- Vásquez-Garzón, V.R.; Arellanes-Robledo, J.; García-Román, R.; Aparicio-Rautista, D.I.; Villa-Treviño, S. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free. Radic. Res. 2009, 43, 128–137. [Google Scholar] [CrossRef]
- Cebecioglu, R.; Yildirim, M.; Akagunduz, D.; Korkmaz, I.; Tekin, H.O.; Atasever-Arslan, B.; Catal, T. Synergistic effects of quercetin and selenium on oxidative stress in endometrial adenocarcinoma cells. Bratisl. Med. J. 2019, 120, 449–455. [Google Scholar] [CrossRef]
- Yao, S.; Sang, H.; Song, G.; Yang, N.; Liu, Q.; Zhang, Y.; Jiao, P.; Zong, C.; Qin, S. Quercetin protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress-C/EBP homologous protein pathway. Exp. Biol. Med. 2012, 237, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, C.; Yao, P.; Gong, Z. Quercetin Alleviates High-Fat Diet-Induced Oxidized Low-Density Lipoprotein Accumulation in the Liver: Implication for Autophagy Regulation. BioMed Res. Int. 2015, 2015, 607531. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Moon, J.-H.; Tsushida, T.; Nagao, A.; Terao, J. Inhibitory Effect of Quercetin Metabolites and Their Related Derivatives on Copper Ion-Induced Lipid Peroxidation in Human Low-Density Lipoprotein. Arch. Biochem. Biophys. 1999, 372, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Yamaguchi, S.; Shirai, M.; Miyoshi, M.; Moon, J.-H.; Oshima, S.; Inakuma, T.; Tsushida, T.; Kato, Y. Protection by quercetin and quercetin 3-O-β-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free. Radic. Res. 2001, 35, 925–931. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.L.; Tsushida, T.; Terao, J. Inhibition of Mammalian 15-Lipoxygenase-Dependent Lipid Peroxidation in Low-Density Lipoprotein by Quercetin and Quercetin Monoglucosides. Arch. Biochem. Biophys. 1998, 349, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.J.L.; Lamb, J.H.; Verschoyle, R.D.; Howells, L.M.; Butterworth, M.; Lim, C.K.; Ferry, D.; Farmer, P.B.; Gescher, A.J. Characterisation of metabolites of the putative cancer chemopreventive agent quercetin and their effect on cyclo-oxygenase activity. Br. J. Cancer 2004, 91, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Malhotra, A.; Dhawan, D.K. COX-2 as a Potential Target in Chemoprevention of Benzo(a)pyrene Induced Lung Carcinogenesis in Mice-combined Role of Curcumin and Quercetin. Am. J. Biomed. Sci. 2012, 4, 194–203. [Google Scholar] [CrossRef]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Attenuates TNF-Induced Inflammation in Hepatic Cells by Inhibiting the NF-κB Pathway. Nutr. Cancer 2012, 64, 588–598. [Google Scholar] [CrossRef]
- Sun, L.; Xu, G.; Dong, Y.; Li, M.; Yang, L.; Lu, W. Quercetin Protects against Lipopolysaccharide-Induced Intestinal Oxidative Stress in Broiler Chickens through Activation of Nrf2 Pathway. Molecules 2020, 25, 1053. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Beak, S.-Y.; Choi, I.; Sung, J.-S. Quercetin and its metabolites protect hepatocytes against ethanol-induced oxidative stress by activation of Nrf2 and AP-1. Food Sci. Biotechnol. 2017, 27, 809–817. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, Y.; Xue, X.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Research Progress of Quercetin Delivery Systems. Curr. Pharm. Des. 2022, 28, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Britton, R.G.; Mazzoletti, M.; Greaves, P.; Broggini, M.; Brown, K.; Steward, W.P.; Gescher, A.J.; Sale, S. Preclinical Colorectal Cancer Chemopreventive Efficacy and p53-Modulating Activity of 3′,4′,5′-Trimethoxyflavonol, a Quercetin Analogue. Cancer Prev. Res. 2010, 3, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Zu, Y.-J.; Nie, S.-F.; Cao, J.; Wang, Q.; Nie, S.-P.; Deng, Z.-Y.; Xie, M.-Y.; Wang, S. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.-S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.-S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Papp, L.V.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian Thioredoxin Reductase by Some Flavonoids: Implications for Myricetin and Quercetin Anticancer Activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin Reductase Is Irreversibly Modified by Curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Hevia, D.; Mayo, J.C.; Gonzalez-Menendez, P.; Coppo, L.; Lu, J.; Holmgren, A.; Sainz, R.M. Thioredoxin 1 modulates apoptosis induced by bioactive compounds in prostate cancer cells. Redox Biol. 2017, 12, 634–647. [Google Scholar] [CrossRef]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The thioredoxin system and cancer therapy: A review. Cancer Chemother. Pharmacol. 2019, 84, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhu, B.; Ren, C.; Kang, F.; Li, J.; Huang, Z.; Lai, Y.; Peng, S.; Ding, K.; Tian, J.; et al. Discovery of New Monocarbonyl Ligustrazine–Curcumin Hybrids for Intervention of Drug-Sensitive and Drug-Resistant Lung Cancer. J. Med. Chem. 2016, 59, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Huang, J.; Zuo, Y.; Li, B.; Guo, Q.; Cui, B.; Shao, W.; Du, J.; Bu, X. 2a, a novel curcumin analog, sensitizes cisplatin-resistant A549 cells to cisplatin by inhibiting thioredoxin reductase concomitant oxidative stress damage. Eur. J. Pharmacol. 2013, 707, 130–139. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1–26. [Google Scholar] [CrossRef]






| Research Model | Carcinogenic Factor | Effect Exerted by Curcumin | Target of Chemoprevention | Author |
|---|---|---|---|---|
| In vivo | AFB1 | Increased expression and activity of GST, GPx, and Nrf2; decreased expression of CYP3A4, CYP1A2, and CYP1A1 | Liver cancer | [120] |
| In vivo | – | Increased levels of Nrf2, GST, GR, and NAD(P)H: quinone reductase; regulation of iNOS and COX-2 | Lymphoma | [125] |
| In vivo | Fe-NTA | Reduction of lipid peroxidation and oxidative DNA damage | Kidney cancer | [128] |
| In vivo | Arsenic | Reduced oxidative damage to DNA and lipid peroxidation | Cancer of the skin, lung, bladder, liver, kidney | [131] |
| In vitro | 131I | Reduced oxidative stress and genotoxicity | Secondary cancers | [132] |
| In vivo | – | Reduced oxidative stress | Oral cancer | [133] |
| In vitro | TNF-α, H2O2 | Inhibition of NF-κb and AP-1 activity; increased expression of GSH and GCLC | Lung cancer | [137] |
| In vivo | NDMA, Opisthorchis viverrini | Inhibition of COX-2, iNOS, and NF-κb expression, reduction in inflammation and oxidative DNA damage | Cholangiocarcinoma | [138] |
| In vitro | – | Increased levels of HO-1 and Nrf2 | Ovarian cancer | [142] |
| Research Model | Carcinogenic Factor | Effect Exerted by Quercetin | Target of Chemoprevention | Author |
|---|---|---|---|---|
| In vivo/in vitro | Benzo[a]pyrene | Reduction of ROS, oxidative DNA damage, and adducts; regulation of GST | Liver cancer | [158] |
| In vitro | – | Increase in NAD(P)H: quinone reductase activity. | Breast cancer | [160] |
| In vivo | DMH | Induction of DNA repair through the Nrf2 pathway | Colorectal cancer | [161] |
| In vivo | Benzo[a]pyrene | Mitigation of oxidative DNA damage; downregulation of COX-2 | Lung cancer | [162,188] |
| In vitro | UVB | Elimination of ROS, prevention of mitochondrial damage, and lipid peroxidation | Skin cancer | [167] |
| In vivo | – | Reduction of ROS, induction of apoptosis of cancer cells, reduction of PKC activity | Lymphoma | [169] |
| In vitro | LPS | Reduction of ROS and NO | Leukemia | [172] |
| In vivo | Testosterone, MNU | Reduction of lipid peroxidation | Prostate cancer | [179] |
| In vitro | – | Reduction of lipid peroxidation (effect combined with selenium) | Endometrial cancer | [181] |
| In vitro | TNF [181], ethanol [183] | Inhibition of ROS production, COX-2 activity, NF-κb pathway [181], Nrf2 | Liver cancer | [189,192] |
| activation, and HO-1 induction [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecerska-Heryć, E.; Wiśniewska, Z.; Serwin, N.; Polikowska, A.; Goszka, M.; Engwert, W.; Michałów, J.; Pękała, M.; Budkowska, M.; Michalczyk, A.; et al. Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin—A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4505. https://doi.org/10.3390/ijms25084505
Cecerska-Heryć E, Wiśniewska Z, Serwin N, Polikowska A, Goszka M, Engwert W, Michałów J, Pękała M, Budkowska M, Michalczyk A, et al. Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin—A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(8):4505. https://doi.org/10.3390/ijms25084505
Chicago/Turabian StyleCecerska-Heryć, Elżbieta, Zofia Wiśniewska, Natalia Serwin, Aleksandra Polikowska, Małgorzata Goszka, Weronika Engwert, Jaśmina Michałów, Maja Pękała, Marta Budkowska, Anna Michalczyk, and et al. 2024. "Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin—A Comprehensive Review" International Journal of Molecular Sciences 25, no. 8: 4505. https://doi.org/10.3390/ijms25084505
APA StyleCecerska-Heryć, E., Wiśniewska, Z., Serwin, N., Polikowska, A., Goszka, M., Engwert, W., Michałów, J., Pękała, M., Budkowska, M., Michalczyk, A., & Dołęgowska, B. (2024). Can Compounds of Natural Origin Be Important in Chemoprevention? Anticancer Properties of Quercetin, Resveratrol, and Curcumin—A Comprehensive Review. International Journal of Molecular Sciences, 25(8), 4505. https://doi.org/10.3390/ijms25084505