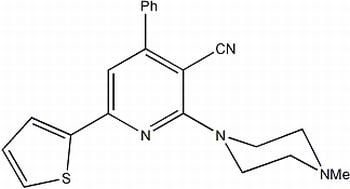
2-(4-Methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile
Abstract
:Introduction
Results and Discussion
Experimental
Synthesis of 2-(4-methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile (4)
Single Crystal X-Ray Crystallographic Data of 2
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Barsoum, F.F. Synthesis and vasodilation activity of some novel bis(3-pyridinecarbonitrile) derivatives. Eur. J. Med. Chem. 2010, 45, 5176–5182. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, P.; Gros, P.C.; Fort, Y. Solid phase synthesis of pyridine-based derivatives from a 2-chloro-5-bromopyridine scaffold. J. Comb. Chem. 2005, 7, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.P.; Mohan, S. Vibrational spectra and normal co-ordinate analysis of 2-aminopyridine and 2-amino picoline. Spectrochim. Acta A 2006, 64, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tumey, L.N.; Bhagirath, N.; Brennan, A.; Brooijmans, N.; Lee, J.; Yang, X.; Boschelli, D.H. 5-Vinyl-3-pyridinecarbonitrile inhibitors of PKCθ: Optimization of enzymatic and functional activity. Bioorg. Med. Chem. 2009, 17, 7933–7948. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Eid, C.; Lee, J.; Liu, E.; Chaudhary, D.; Boschelli, D.H. Synthesis and PKCθ inhibitory activity of a series of 5-vinyl phenyl sulfonamide-3-pyridinecarbonitriles. Bioorg. Med. Chem. Lett. 2009, 19, 6575–6577. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Boschelli, D.H.; Tumey, L.N.; Bhagirath, N.; Subrath, J.; Shim, J.; Wang, Y.; Wu, B.; Eid, C.; Lee, J.; et al. First generation 5-vinyl-3-pyridinecarbonitrile PKCθ inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5829–5832. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Kopf, M. Cell fate and function: PKC-theta and beyond. Trends Immunol. 2008, 29, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-L.; Zhao, J.; Bi, C.; Chen, X.Y.C.; Hepburn, D.L.; Wang, J.; Sedgwick, J.D.; Chintalacharuvu, S.R.; Na, S. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase Cθ-deficient mice. J. Immunol. 2006, 176, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Izmailova, E.; Fitzgerald, M.; Walker, R.; Hattersley, M.; Silva, M.; Siebert, E.; Terkelsen, J.; Picarella, D.; Pickard, M.D.; et al. PKC-theta-deficient mice are protected from Th1-dependent antigen-induced arthritis. J. Immunol. 2006, 177, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, F.F.; Hussein, M.M. New 3-pyridinecarbonitrile derivatives and their antimicrobial properties. Boll. Chim. Farm. 2002, 141, 181–187. [Google Scholar] [PubMed]
- Girgis, A.S.; Kalmouch, A.; Hosni, H.M. Synthesis of novel 3-pyridinecarbonitriles with amino acids function and their fluorescence properties. Amino Acid 2004, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.H.; Tala, S.D.; Akbari, J.D.; Dhaduk, M.F.; Joshi, K.A.; Joshi, H.S. Synthesis and antimicrobial activity of some new cyanopyridine and cyanopyranes towards Mycobacterium tuberculosis and other microoganisms. Ind. J. Chem. 2009, 48B, 833–839. [Google Scholar]
- Adnan, A.B.; Azza, M.B. Novel milrinone analogous of pyridine-3-carbonitrile derivatives as promising cardiotonic agents. Eur. J. Med. Chem. 2005, 40, 1405–1413. [Google Scholar]
- Missori, M.; de Spirito, M.; Ferrari, L.; Selci, S.; Gnoli, A.; Bertani, F.R.; Girgis, A.S.; El-Saied, H.; Basta, A.H. Novel fluorescent security marker. Part I: Morphological and optical properties of 2-amino-6-ethoxy-4-[4-(4-morpholinyl)phenyl]-3,5-pyridinedicarbonitrile nanoparticles. J. Nanopart. Res. 2012, 14, 649–660. [Google Scholar] [CrossRef]
- Basta, A.H.; Girgis, A.S.; El-Saied, H.; Mohamed, M.A. Synthesis of fluorescence active pyridinedicarbonitriles and studying their application in functional paper. Mater. Lett. 2011, 65, 1713–1718. [Google Scholar] [CrossRef]
- Basta, A.H.; Girgis, A.S.; El-Saied, H. Fluorescence behavior of new 3-pyridinecarbonitrile containing compounds and their application in security paper. Dyes Pigm. 2002, 54, 1–10. [Google Scholar] [CrossRef]
- Mishriky, N.; Asaad, F.M.; Ibrahim, Y.A.; Girgis, A.S. New pyridinecarbonitriles from fluoro arylpropenones. Recl. Trav. Chim. Pays-Bas 1994, 113, 35–39. [Google Scholar] [CrossRef]
- Mishriky, N.; Asaad, F.M.; Ibrahim, Y.A.; Girgis, A.S. Synthetic approaches towards 5H-indeno[1,2-b]pyridines. J. Chem. Res. (S) 1997, 316–317. [Google Scholar] [CrossRef]
- Mishriky, N.; Ibrahim, Y.A.; Girgis, A.S.; Fawzy, N.G. Synthetic approaches towards benzo[h]quinoline-3-carbonitriles. Pharmazie 2000, 55, 269–272. [Google Scholar] [PubMed]
- Sharanin, Y.A.; Promonenkov, V.K.; Shestopalov, A.M. Nitrile cyclization reactions. V. 2-Aryl-3-(2-thienoyl)-1,1-dicyanopropanes and derivatives of pyridines made from them. Zh. Org. Khim. 1982, 18, 630–640. [Google Scholar] [CrossRef]
- Greiner-Bechert, L.; Otto, H.-H. 1,4-Pentandien-3-ones, XXXII: Reaction of 2-acetylthiophene and 2-acetylfuran with malononitrile and aldehydes, and synthesis and properties of phenylene-bis[(thienyl/furyl)nicotinonitrile] derivatives. Arch. Pharm. (Weinheim) 1991, 324, 563–572. [Google Scholar] [CrossRef]
- Hanton, R.L.; Moratti, C.S.; Shi Z. Simpson, J. 4,5-Dihydrocyclopenta[b]thiophen-6-one. Acta Cryst. E 2012, 68, o371–o372. [Google Scholar] [CrossRef] [PubMed]
- Al-Youbi, O.A.; Asiri, A.M.; Faidallah, H.M.; Ng, S.W.; Tiekink, R.T. 3-Amino-1-(thiophen-2-yl)-9,10-dihydrophenanthrene-2,4-dicarbonitrile. Acta Cryst. E 2012, 68, o1027–o1028. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Moustafa, A.M. Powder study of propanthioamide dervative C8H6N2S2. Cryst. Res. Technol. 2009, 44, 346–350. [Google Scholar] [CrossRef]
- Miller, R.E.; Nord, F.F. Studies on the chemistry of heterocyclics. XVII. Thiophene polyene acids, aldehydes, and ketones. J. Org. Chem. 1951, 16, 1720–1730. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology; Macromolecular crystallography, Part A; Carter, C.W., Jr., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, 307 M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Mackay, S.; Gilmore, C.J.; Edwards, C.; Stewart, N.; Shankland, K. maXus Computer Program for the Solution and Refinement of Crystal Structures; Bruker Nonius, The Netherlands, MacScience, Japan and The University of Glasgow, 1999. [Google Scholar]
- Johnson, C.K. ORTEP--II. A Fortran Thermal-Ellipsoid Plot Program; Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1976. [Google Scholar]



| Entry | X | Y | z | Ueq | Occ |
|---|---|---|---|---|---|
| S1 | 0.52589(8) | 0.32501(11) | 0.36628(12) | 0.0970(7) | 1.00 |
| O2 | 0.69418(18) | 0.1481(2) | 0.3989(2) | 0.0734(15) | 1.00 |
| C3 | 0.8541(2) | 0.1106(3) | 0.2723(3) | 0.0462(18) | 1.00 |
| N4 | 0.9703(2) | 0.1684(3) | −0.0066(3) | 0.082(2) | 1.00 |
| C5 | 0.8211(3) | −0.1158(4) | 0.2735(3) | 0.064(2) | 1.00 |
| C6 | 0.9616(2) | 0.1392(3) | 0.2462(3) | 0.0486(17) | 1.00 |
| C7 | 0.8107(2) | −0.0058(3) | 0.2039(3) | 0.0495(18) | 1.00 |
| C8 | 0.7870(2) | 0.2243(3) | 0.2392(3) | 0.0523(18) | 1.00 |
| C9 | 0.9662(2) | 0.1547(3) | 0.1028(4) | 0.0549(18) | 1.00 |
| C10 | 0.6988(2) | 0.2233(3) | 0.3113(3) | 0.0497(19) | 1.00 |
| C11 | 1.0333(3) | 0.0438(3) | 0.3056(3) | 0.056(2) | 1.00 |
| N12 | 1.0872(2) | −0.0301(3) | 0.3566(3) | 0.082(2) | 1.00 |
| C13 | 0.7604(3) | −0.0079(4) | 0.0702(3) | 0.067(2) | 1.00 |
| C14 | 0.6229(2) | 0.3184(3) | 0.2777(3) | 0.055(2) | 1.00 |
| C15 | 0.7835(3) | −0.2240(4) | 0.2129(5) | 0.084(3) | 1.00 |
| C16 | 0.7222(3) | −0.1158(6) | 0.0096(4) | 0.093(3) | 1.00 |
| C17 | 0.6139(3) | 0.4093(4) | 0.1841(4) | 0.078(3) | 1.00 |
| C18 | 0.7343(3) | −0.2245(5) | 0.0807(6) | 0.095(3) | 1.00 |
| C19 | 0.5267(4) | 0.4814(4) | 0.1824(4) | 0.105(3) | 1.00 |
| C20 | 0.4737(3) | 0.4462(5) | 0.2756(5) | 0.107(3) | 1.00 |
| Geometrical parameters | Bond lengths (Å); Bond angles (°) |
|---|---|
| S(1)-C(14) | 1.726(3) |
| S(1)-C(20) | 1.682(4) |
| O(2)-C(10) | 1.219(3) |
| C(3)-C(6) | 1.556(3) |
| C(3)-C(7) | 1.506(3) |
| C(3)-C(8) | 1.532(3) |
| N(4)-C(9) | 1.131(3) |
| C(5)-C(7) | 1.383(3) |
| C(5)-C(15) | 1.379(4) |
| C(6)-C(9) | 1.475(4) |
| C(6)-C(11) | 1.466(4) |
| C(7)-C(13) | 1.392(3) |
| C(8)-C(10) | 1.512(3) |
| C(10)-C(14) | 1.449(3) |
| C(11)-N(12) | 1.139(3) |
| C(13)-C(16) | 1.379(4) |
| C(14)-C(17) | 1.360(4) |
| C(15)-C(18) | 1.373(5) |
| C(16)-C(18) | 1.380(5) |
| C(17)-C(19) | 1.411(4) |
| C(19)-C(20) | 1.346(5) |
| C(3)-C(6)-C(9) | 114.2(2) |
| C(9)-C(6)-C(11) | 109.6(2) |
| C(3)-C(6)-C(11) | 110.5(2) |
| C(14)-S(1)-C(20) | 91.6(2) |
| N(4)-C(9)-C(6) | 178.9(3) |
| C(6)-C(11)-N(12) | 177.1(3) |
| D-H….A | D-H (Å) | H…A (Å) | D….A (Å) | D-H….A ° |
|---|---|---|---|---|
| C6-H6-N4 | 0960(2) | 2.431(2) | 3.251(3) | 143.19(20) |
| C8-H8A-N12 | 0.960(3) | 2.658(2) | 3.409(4) | 135.39(20) |
| C17-H17-O2 | 0.960(3) | 2.547(2) | 3.336(3) | 139.58(24) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mishriky, N.; Moustafa, A.M. 2-(4-Methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile. Molbank 2013, 2013, M794. https://doi.org/10.3390/M794
Mishriky N, Moustafa AM. 2-(4-Methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile. Molbank. 2013; 2013(1):M794. https://doi.org/10.3390/M794
Chicago/Turabian StyleMishriky, Nawal, and Aisha M. Moustafa. 2013. "2-(4-Methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile" Molbank 2013, no. 1: M794. https://doi.org/10.3390/M794
APA StyleMishriky, N., & Moustafa, A. M. (2013). 2-(4-Methylpiperazin-1-yl)-4-phenyl-6-(thiophen-2-yl)-pyridine-3-carbonitrile. Molbank, 2013(1), M794. https://doi.org/10.3390/M794