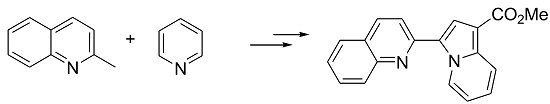
Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate
Abstract
:1. Introduction

2. Experimental Section
2.1. General Information
2.2. Synthesis of Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate (2)
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgements
Author Contributions
Conflicts of Interest
References
- Georgescu, E.; Dumitrascu, F.; Georgescu, F.; Draghici, C.; Barbu, L. A Novel Approach for the Synthesis of 5-Pyridylindolizine Derivatives via 2-(2-Pyridyl)pyridinium Ylides. J. Heterocycl. Chem. 2013, 50, 78–82. [Google Scholar] [CrossRef]
- Malonne, H.; Hanuise, J.; Fontaine, J. Topical Anti-inflammatory Activity of New 2-(1-Indolizinyl)propionic Acid Derivatives in Mice. Pharm. Pharmacol. Commun. 1998, 4, 241–242. [Google Scholar]
- Kitadokoro, K.; Hagishita, S.; Sato, T.; Ohtani, M.; Miki, K. Crystal Structure of Human Secretory Phospholipase A2-IIA Complex with the Potent Indolizine Inhibitor 120–1032. J. Biochem. 1998, 123, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Bolle, L.D.; Andrei, G.; Snoeck, R.; Zhang, Y.; Lommel, A.V.; Otto, M.; Bousseau, A.; Roy, C.; Clercq, E.D.; Naesens, L. Potent, selective and cell-mediated inhibition of human herpesvirus 6 at an early stage of viral replication by the non-nucleoside compound CMV423. Biochem. Pharmacol. 2004, 67, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sonnet, P.; Dallemagne, P.; Guillon, J.; Engueard, C.; Stiebing, S.; Tangue, J.; Bureau, B.; Rault, S.; Auvray, P.; Moslemi, S.; et al. New aromatase inhibitors. Synthesis and biological activity of aryl-substituted pyrrolizine and indolizine derivatives. Bioorg. Med. Chem. 2000, 8, 945–955. [Google Scholar] [CrossRef]
- Campagna, F.; Carotti, A.; Casini, G.; Macripo, M. Synthesis of New Heterocyclic Ring Systems: Indeno[2,1-b]benzo[g]indolizine and indeno[1′,2′:5,4]pyrrolo[2,1-a]phthalazine. Heterocycles 1990, 31, 97–107. [Google Scholar] [CrossRef]
- Bols, M.; Lillelund, V.H.; Jensen, H.H.; Liang, X. Recent Developments of Transition-State Analogue Glycosidase Inhibitors of Non-Natural Product Origin. Chem. Rev. 2002, 102, 515–553. [Google Scholar]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron: Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Shipman, M. Indolizines in science of synthesis. In In Fused Five-Membered Hetarenes with One Heteroatom; Thomas, E.J., Ed.; Georg Thieme Verlag: New York, NY, USA, 2000; Volume 10, pp. 745–787. [Google Scholar]
- Agejas, J.; Cuadro, A.M.; Pastor, M.; Vaquero, J.J.; García-Navío, J.L.; Alvarez-Builla, J. N-(Pyridylmethyl)azinium Salts: Precursors of Pyridyl-stabilised Azinium N-Ylides. Tetrahedron 1995, 51, 12425–12438. [Google Scholar] [CrossRef]
- Jaung, J.Y.; Jung, Y.S. 1,3-Dipolar Cycloaddition Reactions of Pyridinium Azomethine Ylides Containing 5,6-Dicyanopyrazines. Bull. Korean Chem. Soc. 2003, 24, 1565–1566. [Google Scholar] [CrossRef]
- Mao, Z.; Li, X.; Lin, X.; Lu, P.; Wang, Y. One-pot multicomponent synthesis of polysubstituted indolizines. Tetrahedron 2012, 68, 85–91. [Google Scholar] [CrossRef]
- Boeklheide, V.; Farenholtz, K. The Formation of Pyrrocolines by the Reaction of Dimethyl Acetylenedicarboxylate with Heterocyclic Zwitterions. J. Am. Chem. Soc. 1961, 83, 458–462. [Google Scholar] [CrossRef]
- Hendrick, C.A.; Ritchie, E.; Taylor, W.C. Pyridinium ylids in synthesis. III. Synthesis of indolizines. Aust. J. Chem. 1967, 20, 2467–2477. [Google Scholar] [CrossRef]
- Padwa, A.; Austin, D.J.; Precedo, L.; Zhi, L. Cycloaddition reactions of pyridinium and related azomethine ylides. J. Org. Chem. 1993, 58, 1144–1150. [Google Scholar] [CrossRef]
- Belguedj, R.; Bouraiou, A.; Bouacida, S.; Merazig, H.; Chibani, A. Pyridinium ylides in the one-pot synthesis of a new quinoline/indolizine hybrid. Z. Naturforsch. 2015, 70, 885–887. [Google Scholar] [CrossRef]
- Belguedj, R.; Bouacida, S.; Merazig, H.; Belfaitah, A.; Bouraiou, A. 1-(2′-Benzimidazolylmethyl)-pyridinium ylide in the one-pot synthesis of indolizine and benzimidazo[1,2-a]pyridine derivatives. Z. Naturforsch. 2015, 70, 555–561. [Google Scholar] [CrossRef]
- Albright, J.D.; Shepherd, R.G. Reactions of 1,2-Dimethyl-5-nitroimidazole, novel methods of conversion of the 2-Methyl group to a nitrile. J. Heterocycl. Chem. 1973, 10, 899–907. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belguedj, R.; Lamera, E.; Bouraiou, A.; Bouaziz, Z.; Chibani, A. Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate. Molbank 2016, 2016, M883. https://doi.org/10.3390/M883
Belguedj R, Lamera E, Bouraiou A, Bouaziz Z, Chibani A. Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate. Molbank. 2016; 2016(1):M883. https://doi.org/10.3390/M883
Chicago/Turabian StyleBelguedj, Roumaissa, Esma Lamera, Abdelmalek Bouraiou, Zouhair Bouaziz, and Aissa Chibani. 2016. "Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate" Molbank 2016, no. 1: M883. https://doi.org/10.3390/M883
APA StyleBelguedj, R., Lamera, E., Bouraiou, A., Bouaziz, Z., & Chibani, A. (2016). Methyl 3-(Quinolin-2-yl)indolizine-1-carboxylate. Molbank, 2016(1), M883. https://doi.org/10.3390/M883