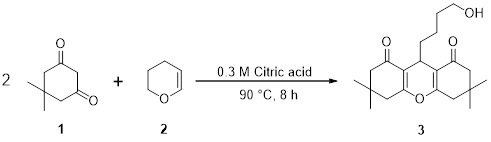
9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione
Abstract
:1. Introduction
2. Results and Discussion


3. Experimental Section
3.1. General Information
3.2. Synthesis of 9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione
Supplementary Materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casillas, L.K.; Townsend, C.A. Total synthesis of O-methylsterigmatocystin using N-alkylnitrilium salts and carbonyl−alkene interconversion in a new xanthone synthesis. J. Org. Chem. 1999, 64, 4050–4059. [Google Scholar] [CrossRef]
- Piettre, A.; Chevenier, E.; Massardier, C.; Gimbert, Y.; Greene, A.E. Synthetic approach to hypoxyxylerone, novel inhibitor of topoisomerase I. Org. Lett. 2002, 4, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Fukuta, Y.; Ota, S.; Kamiya, M.; Sato, M. Xanthone natural products via N-heterocyclic carbene catalysis: Total synthesis of atroviridin. J. Org. Chem. 2011, 76, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.W.; Martin, J.A.; Merrett, J.H.; Parkes, K.E.B.; Thomas, G.J. Pyrimidine Nucleosides. PCT Int. Appl. WO 9706178, 24 July 1997. [Google Scholar]
- Hideo, T.; Teruomi, J. 1-Benzopyrano[2,3-b]xanthene Derivative and Its Preparation. Jpn. Pat. 56005480, 20 January 1981. [Google Scholar]
- Renno, R.Z.; Miller, J.W. Photosensitizer delivery for photodynamic therapy of choroidal neovascularization. Adv. Drug Deliv. Rev. 2001, 52, 63–78. [Google Scholar] [CrossRef]
- Ahmad, M.; King, T.A.; Ko, D.-K.; Cha, B.H.; Lee, J. Performance and photostability of xanthene and pyrromethene laser dyes in sol–gel phases. J. Phys. D: Appl. Phys. 2002, 35, 1473–1476. [Google Scholar] [CrossRef]
- Iniyavan, P.; Sarveswari, S.; Vijayakumar, V. Synthesis and antioxidant studies of novel bi-, tri-, and tetrapodal 9-aryl-1,8-dioxo-octahydroxanthenes. Tetrahedron Lett. 2015, 56, 1401–1406. [Google Scholar] [CrossRef]
- Kumar, G.S.S.; Prabhu, A.A.M.; Seethalashmi, P.G.; Bhuvanesh, N.; Kumaresan, S. Self-catalyzed syntheses, structural characterization, dpph radical scavenging-, cytotoxicity-, and dft studies of phenoxyaliphatic acids of 1,8-dioxo-octahydroxanthene derivatives. J. Mol. Struct. 2014, 1059, 51–60. [Google Scholar] [CrossRef]
- Mulakayala, N.; Murthy, P.V.; Rambabu, D.; Aeluri, M.; Adepu, R.; Krishna, G.R.; Reddy, C.M.; Prasad, K.R.; Chaitanya, M.; Kumar, C.S.; et al. Catalysis by molecular iodine: A rapid synthesis of 1,8-dioxo-octahydroxanthenes and their evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Ali, I.; Shah, M.R.; Badshah, A.; Qayum, M.; Khan, H.; Khan, I.; Ali, S. Amberlite ir-120h as a recyclable catalyst for the synthesis of 1,8-dioxo-octahydroxanthene analogs and their evaluation as potential leishmanicidal agents. RSC Adv. 2013, 3, 21753–21758. [Google Scholar] [CrossRef]
- Ulusal, H.; Fındıkkıran, G.; Demirkol, O.; Akbaşlar, D.; Giray, E.S. Supercritical diethylether: A novel solvent for the synthesis of aryl-3,4,5,6,7,9-hexahydroxanthene-1,8-diones. J. Supercrit. Fluids 2015, 105, 146–150. [Google Scholar] [CrossRef]
- He, F.; Li, P.; Gu, Y.; Li, G. Glycerol as a promoting medium for electrophilic activation of aldehydes: Catalyst-free synthesis of di(indolyl)methanes, xanthene-1,8(2h)-diones and 1-oxo-hexahydroxanthenes. Green Chem. 2009, 11, 1767–1773. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Arzroomchilar, S. 1-Methylimidazolium triflouroacetate ([hmim]tfa): An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Catal. Commun. 2008, 9, 939–942. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, B.; Li, M.; Gu, Y. Gluconic acid aqueous solution: A task-specific bio-based solvent for ring-opening reactions of dihydropyrans. Tetrahedron 2013, 69, 1057–1064. [Google Scholar] [CrossRef]
- Bigdeli, M. Clean synthesis of 1,8-dioxooctahydroxanthenes promoted by dabco-bromine in aqueous media. Chin. Chem. Lett. 2010, 21, 1180–1182. [Google Scholar] [CrossRef]
- Das, B.; Kashanna, J.; Kumar, R.A.; Jangili, P. Efficient organocatalytic synthesis of 1,8-dioxo-octahydroxanthenes. Synth. Commun. 2012, 42, 2876–2884. [Google Scholar] [CrossRef]
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, V.S.; Rao, Y.K. Amberlyst-15: An efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. J. Mol. Catal. A: Chem. 2006, 247, 233–239. [Google Scholar] [CrossRef]
- Javid, A.; Heravi, M.M.; Bamoharram, F.F. One-pot synthesis of 1,8-dioxo-octahydroxanthenes utilizing silica-supported preyssler nano particles as novel and efficient reusable heterogeneous acidic catalyst. E-J. Chem. 2011, 8, 910–916. [Google Scholar] [CrossRef]
- Niknam, K.; Panahi, F.; Saberi, D.; Mohagheghnejad, M. Silica-bonded S-sulfonic acid as recyclable catalyst for the synthesis of 1,8-dioxo-decahydroacridines and 1,8-dioxo-octahydroxanthenes. J. Heterocycl. Chem. 2010, 47, 292–300. [Google Scholar]
- Dadhania, A.N.; Patel, V.K.; Raval, D.K. Catalyst-free sonochemical synthesis of 1,8-dioxo-octahydroxanthene derivatives in carboxy functionalized ionic liquid. C. R. Chim. 2012, 15, 378–383. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Amiri, G.; Sepehrian, H. Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media. Ultrason. Sonochem. 2010, 17, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Gao, Y.; Miao, C.; Zhu, S.; Li, T.; Zhang, X.; Shi, D. The reaction of aromatic dialdehyde with dimedone under microwave irradiation. Synth. Commun. 2004, 34, 2617–2622. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.-J. Domino reaction of anilines with 3,4-dihydro-2H-pyran catalyzed by cation-exchange resin in water: An efficient synthesis of 1,2,3,4-tetrahydroquinoline derivatives. Green Chem 2003, 5, 627–629. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, C.-J. InCl3-catalyzed reaction of aromatic amines with cyclic hemiacetals in water: Facile synthesis 1,2,3,4-tetrahydroquinoline derivatives. Tetrahedron Lett. 2003, 44, 153–156. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, C.A.; Sierra, C.; Ochoa-Puentes, C. 9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione. Molbank 2016, 2016, M884. https://doi.org/10.3390/M884
Navarro CA, Sierra C, Ochoa-Puentes C. 9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione. Molbank. 2016; 2016(1):M884. https://doi.org/10.3390/M884
Chicago/Turabian StyleNavarro, Camilo A., Cesar Sierra, and Cristian Ochoa-Puentes. 2016. "9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione" Molbank 2016, no. 1: M884. https://doi.org/10.3390/M884
APA StyleNavarro, C. A., Sierra, C., & Ochoa-Puentes, C. (2016). 9-(4-Hydroxybutyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione. Molbank, 2016(1), M884. https://doi.org/10.3390/M884