Cis,exo-1,2,3,4,4a,13b-hexahydro-1,4-methano-5-isopropoxy-9H-tribenzo[b,f]azepine
Abstract
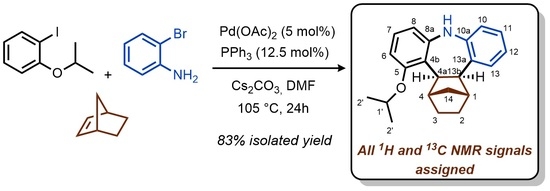
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Daugulis, O.; Do, H.-Q.; Shabashov, D. Palladium-and copper-catalyzed arylation of carbon−hydrogen bonds. Acc. Chem. Res. 2009, 42, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Towards mild metal-catalyzed C-H bond activation. Chem. Soc. Rev. 2011, 40, 4740–4761. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, S.R.; Sanford, M.S. Controlling site selectivity in palladium-catalyzed C-H bond functionalization. Acc. Chem. Res. 2012, 45, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Rouquet, G.; Chatani, N. Catalytic Functionalization of C(sp2)-H and C(sp3)-H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, W.I.; Jun, C.H. Metal-Organic Cooperative Catalysis in C-H and C-C Bond Activation. Chem. Rev. 2017, 117, 8977–9015. [Google Scholar] [CrossRef] [PubMed]
- Catellani, M.; Frignani, F.; Rangoni, A. A Complex Catalytic Cycle Leading to a Regioselective Synthesis of o,o′-Disubstituted Vinylarenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 119–122. [Google Scholar] [CrossRef]
- Della Ca’, N.; Fontana, M.; Motti, E.; Catellani, M. Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C-H Bond Activation. Acc. Chem. Res. 2016, 49, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lautens, M. Palladium-catalysed norbornene-mediated C-H functionalization of arenes. Nat. Chem. 2015, 7, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Faccini, F.; Motti, E.; Catellani, M. A New Reaction Sequence Involving Palladium-Catalyzed Unsymmetrical Aryl Coupling. J. Am. Chem. Soc. 2004, 126, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Motti, E.; Faccini, F.; Ferrari, I.; Catellani, M.; Ferraccioli, R. Sequential Unsymmetrical Aryl Coupling of o-Substituited Aryl Iodides with o-Bromophenols and Reaction with Olefins: Palladium-Catalyzed Synthesis of 6H-Dibenzopyran Derivatives. Org. Lett. 2006, 8, 3967–3970. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Sassi, G.; Catellani, M. A Direct Palladium-Catalyzed Route to Selectively Substituted Carbazoles through Sequential C-C and C-N Bond Formation: Synthesis of Carbazomycin A. Adv. Synth. Catal. 2008, 350, 2179–2182. [Google Scholar] [CrossRef]
- Della Ca’, N.; Motti, E.; Catellani, M. Palladium-Catalyzed Synthesis of Selectively Substituted Phenanthridine Derivatives. Adv. Synth. Catal. 2008, 350, 2513–2516. [Google Scholar] [CrossRef]
- Maestri, G.; Della Ca’, N.; Catellani, M. A catalytic synthesis of selectively substituted biaryls through sequential intermolecular coupling involving arene and ketone C-H bond functionalization. Chem. Commun. 2009, 4892–4894. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Maestri, G.; Catellani, M. Palladium/Norbornene-Catalyzed Synthesis of Heteroatom-Containing o-Teraryls from Aryl Iodides and Heteroarenes through Double C-H Activation in Sequence. Chem. Eur. J. 2009, 15, 7850–7853. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Motti, E.; Mega, A.; Catellani, M. One-Pot Palladium-Catalyzed Synthesis of Selectively Substituted Phenanthridines by Sequential Aryl-Aryl and Heck Couplings, Aza-Michael and Retro-Mannich Reactions. Adv. Synth. Catal. 2010, 352, 1451–1454. [Google Scholar] [CrossRef]
- Motti, E.; Della Ca’, N.; Deledda, S.; Fava, E.; Panciroli, F.; Catellani, M. Palladium-catalyzed unsymmetrical aryl couplings in sequence leading to o-teraryls: Dramatic olefin effect on selectivity. Chem. Commun. 2010, 46, 4291–4293. [Google Scholar] [CrossRef] [PubMed]
- Motti, E.; Della Ca’, N.; Xu, D.; Piersimoni, A.; Bedogni, E.; Zhou, Z.-M.; Catellani, M. A Sequential Pd/Norbornene-Catalyzed Process Generates o-Biaryl Carbaldehydes or Ketones via a Redox Reaction or 6H-Dibenzopyrans by C-O Ring Closure. Org. Lett. 2012, 14, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- Motti, E.; Della Ca’, N.; Xu, D.; Armani, S.; Aresta, B.M.; Catellani, M. Competitive pathways in Pd-catalyzed synthesis of arylphenols. Tetrahedron 2013, 69, 4421–4428. [Google Scholar] [CrossRef]
- Xu, D.; Dai, L.; Catellani, M.; Motti, E.; Della Ca’, N.; Zhou, Z. A Novel Enantioselective Synthesis of 6H-Dibenzopyran Derivatives by Combined Palladium/Norbornene and Cinchona Alkaloid Catalysis. Org. Biomol. Chem. 2015, 13, 2260–2263. [Google Scholar] [CrossRef] [PubMed]
- Della Ca’, N.; Fontana, M.; Xu, D.; Cremaschi, M.; Lucentini, R.; Zhou, Z.-M.; Catellani, M.; Motti, E. Formation of a Carbonyl Group ortho to a Biaryl Structure or a 6H-Dibenzopyran by a Palladium/Norbornene-catalyzed Ordered Reaction Sequence. Tetrahedron 2015, 71, 6389–6401. [Google Scholar] [CrossRef]
- Della Ca’, N.; Maestri, G.; Malacria, M.; Derat, E.; Catellani, M. Palladium-Catalyzed Reaction of Aryl Iodides with ortho-Bromoanilines and Norbornene/Norbornadiene: Unexpected Formation of Dibenzoazepine Derivatives. Angew. Chem. Int. Ed. 2011, 50, 12257–12261. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M.A.; Kasper, S. A review of the evidence for carbamazepine and oxcarbazepine in the treatment of bipolar disorder. Int. J. Neuropsychopharmacol. 2004, 7, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arguelles, J.M.; Dorado, R.; Sepulveda, J.M.; Huet, R.; Arrojo, F.G.; Aragon, E.; Herrera, A.; Trron, C.; Anciones, B. Oxcarbazepine monotherapy in carbamazepine-unresponsive trigeminal neuralgia. J. Clin. Neurosci. 2008, 15, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, S.; Lang, M.; Dreker, T.; Kraus, J.; Hamm, S.; Meere, C.; Feurle, J.; Tasler, S.; Prütting, S.; Kuras, Z.; et al. Inhibitors of potassium channels KV1.3 and IK-1 as immunosuppressants. Bioorg. Med. Chem. Lett. 2009, 19, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.S.; Sheikh, T.; Ho, A.L.; Schwartz, G.K. PTEN Regulates Sensitivity of Melanoma Cells to RO4929097, the γ-Secretase Inhibitor. Anticancer Res. 2013, 33, 1307–1316. [Google Scholar] [PubMed]
- The isopropoxy group can be readily converted into OH group under mild reaction conditions by various methods (AlCl3, diisopropyl-carbodiimide/AlI3, BCl3, TsOH·H2O, TiCl4, Pd/C/H2). For example, isopropyl group was removed quantitatively by BCl3 at 0 °C in 2 h (Lee, Y.-T.; Fong, T.-H.; Chen, H.-M.; Chang, C.-Y.; Wang, Y.-H.; Chern, C.-Y.; Chen, Y.-H. Toxicity Assessments of Chalcone and Some Synthetic Chalcone Analogues in a Zebrafish Model. Molecules 2014, 19, 641–650, doi:10.3390/molecules19010641).




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casnati, A.; Motti, E.; Ca’, N.D. Cis,exo-1,2,3,4,4a,13b-hexahydro-1,4-methano-5-isopropoxy-9H-tribenzo[b,f]azepine. Molbank 2018, 2018, M988. https://doi.org/10.3390/M988
Casnati A, Motti E, Ca’ ND. Cis,exo-1,2,3,4,4a,13b-hexahydro-1,4-methano-5-isopropoxy-9H-tribenzo[b,f]azepine. Molbank. 2018; 2018(1):M988. https://doi.org/10.3390/M988
Chicago/Turabian StyleCasnati, Alessandra, Elena Motti, and Nicola Della Ca’. 2018. "Cis,exo-1,2,3,4,4a,13b-hexahydro-1,4-methano-5-isopropoxy-9H-tribenzo[b,f]azepine" Molbank 2018, no. 1: M988. https://doi.org/10.3390/M988
APA StyleCasnati, A., Motti, E., & Ca’, N. D. (2018). Cis,exo-1,2,3,4,4a,13b-hexahydro-1,4-methano-5-isopropoxy-9H-tribenzo[b,f]azepine. Molbank, 2018(1), M988. https://doi.org/10.3390/M988