1-Adamantylamidoxime
Abstract
:1. Introduction
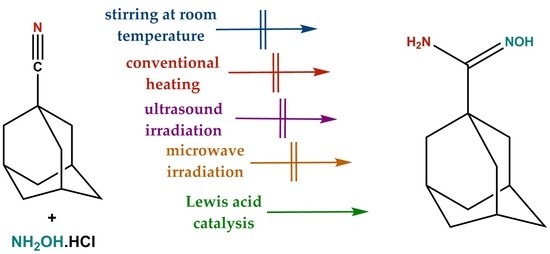
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis
1-Adamantylamidoxime (3)

Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 11β-HSD1 | 11β-hydroxysteroid dehydrogenase type 1 |
| IR | infrared spectroscopy |
| NMR | nuclear magnetic resonance spectroscopy |
| HRMS | high-resolution mass spectroscopy |
| TMS | tetramethylsilane |
References
- Seckl, J.R.; Walker, B.R. Minireview: 11beta-hydroxysteroid dehydrogenase type 1—A tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Staab, C.A.; Maser, E. 11beta-hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J. Steroid Biochem. Mol. Biol. 2010, 119, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.J.; Walker, B.R. 11 beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol. Cell. Endocrinol. 2004, 215, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Baudrand, R.; Dominguez, J.M.; Carvajal, C.A.; Riquelme, A.; Campino, C.; Macchiavello, S.; Bozinovic, M.; Morales, M.; Pizarro, M.; Solis, N.; et al. Overexpression of hepatic 5α-reductase and 11β-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue is associated with hyperinsulinemia in morbidly obese patients. Metabolism 2011, 60, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.M.; Paterson, J.M.; Masuzaki, H.; Holmes, M.C.; Staels, B.; Fievet, C.; Walker, B.R.; Flier, J.S.; Mullins, J.J.; Seckl, J.R. Novel adipose tissue—Mediated resistance to diet-induced visceral obesity in 11β-hydroxysteroid dehydrogenase type 1—Deficient mice. Diabetes 2004, 53, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Lagos, C.F.; Vecchiola, A.; Allende, F.; Fuentes, C.A.; Tichauer, J.E.; Valdivia, C.; Solari, S.; Campino, C.; Tapia-Castillo, A.; Baudrand, R.; et al. Identification of novel 11β-hsd1 inhibitors by combined ligand- and structure-based virtual screening. Mol. Cell. Endocrinol. 2014, 384, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Cadoni, R.; De Luca, L. A fast and efficient one-pot microwave assisted synthesis of variously di-substituted 1,2,4-oxadiazoles. Org. Biomol. Chem. 2011, 9, 7539–7546. [Google Scholar] [CrossRef] [PubMed]
- Eloy, F.; Lenaers, R. The chemistry of amidoximes and related compounds. Chem. Rev. 1962, 62, 155–183. [Google Scholar] [CrossRef]
- Kandre, S.; Bhagat, P.R.; Sharma, R.; Gupte, A. Microwave assisted synthesis of 3,5-disubstituted 1,2,4-oxadiazoles from substituted amidoximes and benzoyl cyanides. Tetrahedron Lett. 2013, 54, 3526–3529. [Google Scholar] [CrossRef]
- Xia, G.; You, X.; Liu, L.; Liu, H.; Wang, J.; Shi, Y.; Li, P.; Xiong, B.; Liu, X.; Shen, J. Design, synthesis and sar of piperidyl-oxadiazoles as 11β-hydroxysteroid dehydrogenase 1 inhibitors. Eur. J. Med. Chem. 2013, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vorona, S.; Artamonova, T.; Zevatskii, Y.; Myznikov, L. An improved protocol for the preparation of 5-substituted tetrazoles from organic thiocyanates and nitriles. Synthesis 2014, 46, 781–786. [Google Scholar]
- Cantillo, D.; Gutmann, B.; Kappe, C.O. An experimental and computational assessment of acid-catalyzed azide-nitrile cycloadditions. J. Org. Chem. 2012, 77, 10882–10890. [Google Scholar] [CrossRef] [PubMed]


| Entry | Reaction Conditions | Base Employed | Nitrile Concentration (g/100 mL) | Reactant Equivalents Relative to Nitrile | Reaction Time | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | Stirring, rt | Na2CO3 | 2 | a: 1, b: 1 | Overnight | 0 |
| 2 | Ultrasound | Na2CO3 | 2 | a: 1, b: 1 | 45 min | 0 |
| 3 | Microwave | Na2CO3 | 2 | a: 1, b: 1 | 1 h | 0 |
| 4 | Reflux | Na2CO3 | 1 | a: 2, b: 2 | 48 h | 6.5 |
| 5 | Reflux | KOH | 2 | a: 2, c: 2 | 25 h | 0 |
| 6 | Reflux, ZnCl2 | Na2CO3 | 1 | a: 1.2, b: 1.2, d: 1 | 17 h | 2.9 |
| 7 | Microwave, ZnCl2 | Na2CO3 | 1 | a: 1, b: 1, d: 0.4 | 1 h | 0 |
| 8 | Reflux, AlCl3 | Na2CO3 | 1 | a: 3, b: 3, e: 0.15 | 15 h | 29 |
| 9 | Reflux, AlCl3 | Na2CO3 | 0.4 | a: 3, b: 3, e: 0.15 | 10 h | 12 |
| 10 | Reflux, AlCl3 | Na2CO3 | 2 | a: 3, b: 3, e: 0.15 | 10 h | 70 |
| 11 | Reflux, AlCl3 | Na2CO3 | 4 | a: 3, b: 3, e: 0.15 | 10 h | 85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diethelm, B.; Lagos, C.F.; Recabarren-Gajardo, G. 1-Adamantylamidoxime. Molbank 2018, 2018, M992. https://doi.org/10.3390/M992
Diethelm B, Lagos CF, Recabarren-Gajardo G. 1-Adamantylamidoxime. Molbank. 2018; 2018(2):M992. https://doi.org/10.3390/M992
Chicago/Turabian StyleDiethelm, Benjamín, Carlos F. Lagos, and Gonzalo Recabarren-Gajardo. 2018. "1-Adamantylamidoxime" Molbank 2018, no. 2: M992. https://doi.org/10.3390/M992
APA StyleDiethelm, B., Lagos, C. F., & Recabarren-Gajardo, G. (2018). 1-Adamantylamidoxime. Molbank, 2018(2), M992. https://doi.org/10.3390/M992