N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide
Abstract
:1. Introduction
2. Results and Discussion
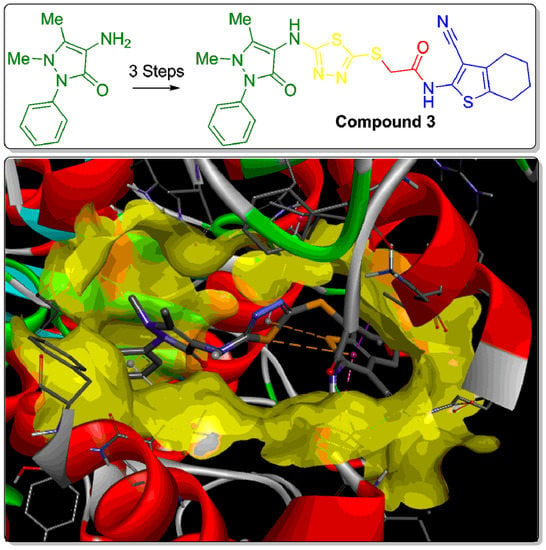
2.1. Synthesis of the Title Compound 3
2.2. Molecular Docking
3. Materials and Methods
3.1. General Information and Compound 3 Synthesis
3.2. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahoo, J.; Sahoo, C.R.; Nandini Sarangi, P.K.; Prusty, S.K.; Padhy, R.N.; Paidesetty, S.K. Molecules with versatile biological activities bearing antipyrinyl nucleus as pharmacophore. Eur. J. Med. Chem. 2020, 186, 111911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef]
- Elattar, K.M.; Fadda, A.A. Chemistry of antipyrine. Synth. Commun. 2016, 46, 1567–1594. [Google Scholar] [CrossRef]
- Vyas, K.M.; Devkar, R.V.; Prajapati, A.; Jadeja, R.N. Pyrazolone incorporating bipyridyl metallointercalators as effective DNA, protein and lung cancer targets: Synthesis, characterization and in vitro biocidal evaluation. Chem. Biol. Interact. 2015, 240, 250–266. [Google Scholar] [CrossRef]
- Gediz Erturk, A.; Omerustaoglu, H. Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives. Molecules 2020, 25, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017 (accessed on 19 April 2021).
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.P.; Kabir, S.N.; Mohamed, T. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Progress in Small Molecule Drug Development. Pharmaceuticals 2010, 3, 1530–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorucci, S.; Distrutti, E. COXIBs, CINODs and H₂S-releasing NSAIDs: Current perspectives in the development of safer non- steroidal anti-inflammatory drugs. Curr. Med. Chem. 2011, 18, 3494–3505. [Google Scholar] [CrossRef] [PubMed]
- Rostom, S.A.; el-Ashmawy, I.M.; Abd el Razik, H.A.; Badr, M.H.; Ashour, H.M. Design and synthesis of some thiazolyl and thiadiazolyl derivatives of antipyrine as potential non-acidic anti-inflammatory, analgesic and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Pannuzzo, G.; Santagati, A.; Catalfo, A.; De Guidi, G.; Cardile, V. Molecular docking and fluorescence characterization of benzothieno[3,2-d]pyrimidin-4-one sulphonamide thio-derivatives, a novel class of selective cyclooxygenase-2 inhibitors. Molecules 2014, 19, 6106–6122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Sayed, M.T.; El-Sharief, M.A.M.S.; Zarie, E.S.; Morsy, N.M.; Elsheakh, A.R.; Voronkov, A.; Berishvili, V.; Hassan, G.S. Design, synthesis, anti-inflammatory activity and molecular docking of potential novel antipyrine and pyrazolone analogs as cyclooxygenase enzyme (COX) inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Holota, S.M.; Nektegayev, I.O.; Soronovych, I.I.; Chubuchna, I.I.; Kolishetska, M.A.; Sysak, S.P.; Regeda, M.S.; Lesyk, R.B. The novel pyrazolin-5-one bearing thiazolidin-4-ones: Synthesis, characterization and biological evaluation. Biopolym. Cell. 2021, 37, 46–61. [Google Scholar] [CrossRef]
- Perrone, M.G.; Centonze, A.; Miciaccia, M.; Ferorelli, S.; Scilimati, A. Cyclooxygenase Inhibition Safety and Efficacy in Inflammation-Based Psychiatric Disorders. Molecules 2020, 25, 5388. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesyk, R.; Vladzimirska, O.; Zimenkovsky, B.; Horishny, V.; Nektegayev, I.; Solyanyk, V.; Vovk, O. New thiazolidones-4 with pyrazolone-5 substituent as the potential NSAIDs. Boll. Chim. Farm. 1998, 137, 210–217. [Google Scholar] [PubMed]
- Patrono, C. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, Y.V.; Obushak, M.D.; Matiychuk, V.S.; Naskrent, M.; Gzella, A.K. A convenient method for the synthesis of 2-[(5-benzyl-1,3-thiazol-2-yl)imino]-1,3-thiazolidin-4-one derivatives. Tetrahedron Lett. 2012, 53, 543–545. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comp. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.; Hornig, M.; Pergola, C.; Meindl, N.; Franke, L.; Tanrikulu, Y.; Dodt, G.; Schneider, G.; Steinhilber, D.; Werz, O. The molecular mechanism of the inhibition by licofelone of the biosynthesis of 5-lipoxygenase products. Br. J. Pharmacol. 2007, 152, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Compounds | COX-2 (PDB:3LN1) | 5-LOX (PDB:3V99) | ||
|---|---|---|---|---|
| Binding Energy (Kcal/mol) | Ki Inhibition Constant | Binding Energy (Kcal/mol) | Ki Inhibition Constant | |
| 2 | −8.5 | 807.43 nM | −6.5 | 11.77 uM |
| 3 | −3.6 | 925.65 µM | −9.0 | 243.23 nM |
| Celecoxib | −12.3 | 12.23 nM | - | - |
| Licofelone | - | - | -8.73 | 443.88 nM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holota, S.; Yushyn, I.; Khyluk, D.; Vynnytska, R.; Lesyk, R. N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide. Molbank 2021, 2021, M1211. https://doi.org/10.3390/M1211
Holota S, Yushyn I, Khyluk D, Vynnytska R, Lesyk R. N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide. Molbank. 2021; 2021(2):M1211. https://doi.org/10.3390/M1211
Chicago/Turabian StyleHolota, Serhii, Ihor Yushyn, Dmytro Khyluk, Renata Vynnytska, and Roman Lesyk. 2021. "N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide" Molbank 2021, no. 2: M1211. https://doi.org/10.3390/M1211
APA StyleHolota, S., Yushyn, I., Khyluk, D., Vynnytska, R., & Lesyk, R. (2021). N-(3-Cyano-4,5,6,7-tetrahydrobenzothiophen-2-yl)-2-[[5-[(1,5-dimethyl-3-oxo-2-phenylpyrazol-4-yl)amino]-1,3,4-thiadiazol-2-yl]sulfanyl]acetamide. Molbank, 2021(2), M1211. https://doi.org/10.3390/M1211