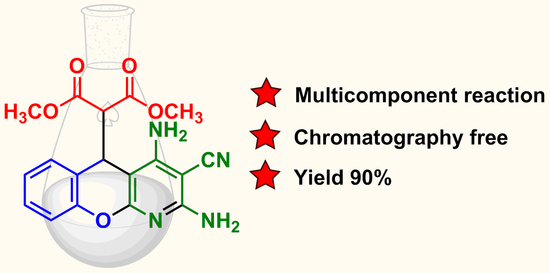
Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Multicomponent Synthesis of Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.-F.W.; Xiang, J.-C.; Gao, Q.-H.; Wu, A.-X. The Applications of DMSO. In Solvents as Reagents in Organic Synthesis: Reactions and Applications; Wu, X.-F.W., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; Chapter 7; pp. 315–353. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Khanaposhtani, M.M.; Larijani, B.; Mahdavi, M. Dimethyl Sulfoxide: Yesterday’s Solvent, Today’s Reagent. Adv. Synth. Catal. 2020, 362, 65–86. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, A.D.; Koketsu, M. 1,3-Selenazoles. In Comprehensive Heterocyclic Chemistry IV, 4th ed.; Black, D.S., Cossy, J., Stevens, C.V., Eds.; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 2022; Chapter 4.08; pp. 685–712. [Google Scholar] [CrossRef]
- Brahmachari, G. Green synthetic approaches for biologically relevant heterocycles: Advanced synthetic techniques—An overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles, Volume 1: Advanced Synthetic Techniques, 2nd ed.; Brahmachari, G., Ed.; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 2021; Chapter 1; pp. 1–8. [Google Scholar] [CrossRef]
- Domling, A.; Wang, W.; Wang, K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoneim, A.A.; El-Farargy, A.F.; Abdelaziz, S. Synthesis and Antimicrobial Activities of New S-Nucleosides of Chromeno[2,3-b]Pyridine Derivatives and C-Nucleosides of [1,2,4]Triazolo[1,5-a]Quinoline Derivatives. Nucleosides Nucleotides Nucleic Acids 2014, 33, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, S.; Pontes, O.; Lopes, D.; Sampaio-Marques, B.; Costa, M.D.; Carvalho, L.; Gonçalves, C.S.; Costa, B.M.; Maciel, P.; Ludovico, P.; et al. Unravelling the anticancer potential of functionalized chromeno[2,3-b]pyridines for breast cancer treatment. Bioorg. Chem. 2020, 100, 103942. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Goto, K.; Terasawa, M. Method for Treatment of Rheumatism. Ger. Offen. DE 3010751, 6 August 1981. Available online: https://worldwide.espacenet.com/patent/search/family/011577010/publication/DE3010751A1?q=DE%203010751%2019810806 (accessed on 13 December 2021).
- Ukawa, K.; Ishiguro, T.; Kuriki, H.; Nohara, A. Synthesis of the metabolites and degradation products of 2-amino-7-isopropyl-5-oxo-5H-(1)benzopyrano(2,3-b)pyridine-3-carboxylic acid (Amoxanox). Chem. Pharm. Bull. 1985, 33, 4432–4437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oset-Gasque, M.J.; González, M.P.; Pérez-Peña, J.; García-Font, N. Toxicological and pharmacological evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines {11-amino-12-aryl-8,9,10,12-tetrahydro-7H-chromeno[2,3-b]quinolin-3-ols} for the potential prevention and treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 74, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, K.; Yaoka, O.; Oe, T. Hypotenseurs. PCT International Application WO 1984001711A1, 10 May 1984. [Google Scholar]
- Verma, C.; Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.; Quraishi, M.A. 2,4-Diamino-5-(phenylthio)-5H-chromeno [2,3-b]pyridine-3-carbonitriles as green and effective corrosion inhibitors: Gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 2016, 6, 53933–53948. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. A facile and efficient multicomponent approach to 5-[5-hydroxy- 3-(trifluoromethyl)-1H-pyrazol-4-yl]-5H-chromeno[2,3-b]pyridines. J. Fluor. Chem. 2018, 213, 31–36. [Google Scholar] [CrossRef]
- Ryzhkov, F.V.; Ryzhkova, Y.E.; Elinson, M.N.; Vorobyev, S.V.; Fakhrutdinov, A.N.; Vereshchagin, A.N.; Egorov, M.P. Catalyst-Solvent System for PASE Approach to Hydroxyquinolinone-Substituted Chromeno[2,3-b]pyridines Its Quantum Chemical Study and Investigation of Reaction Mechanism. Molecules 2020, 25, 2573. [Google Scholar] [CrossRef] [PubMed]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Potassium fluoride catalysed multicomponent approach to medicinally privileged 5-[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl] substituted chromeno[2,3-b]pyridine scaffold. Arkivoc 2019, 2, 38–49. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. Multicomponent Synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic Acids in DMSO. Molecules 2021, 26, 6839. [Google Scholar] [CrossRef] [PubMed]
- Patai, S.; Israeli, Y. 411. The kinetics and mechanisms of carbonyl–methylene condensations. Part VII. The reaction of malononitrile with aromatic aldehydes in ethanol. J. Chem. Soc. 1960, 2025–2030. [Google Scholar] [CrossRef]
- Mittelbach, M. An improved and facile synthesis of 2-amino-1,1,3-tricyanopropene. Mon. Chem. Chem. Mon. 1985, 116, 689–691. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Maslov, O.I.; Elinson, M.N. Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate. Molbank 2022, 2022, M1308. https://doi.org/10.3390/M1308
Ryzhkova YE, Maslov OI, Elinson MN. Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate. Molbank. 2022; 2022(1):M1308. https://doi.org/10.3390/M1308
Chicago/Turabian StyleRyzhkova, Yuliya E., Oleg I. Maslov, and Michail N. Elinson. 2022. "Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate" Molbank 2022, no. 1: M1308. https://doi.org/10.3390/M1308
APA StyleRyzhkova, Y. E., Maslov, O. I., & Elinson, M. N. (2022). Dimethyl 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonate. Molbank, 2022(1), M1308. https://doi.org/10.3390/M1308