Throat Patch Variation in Tayra (Eira barbara) and the Potential for Individual Identification in the Field
Abstract
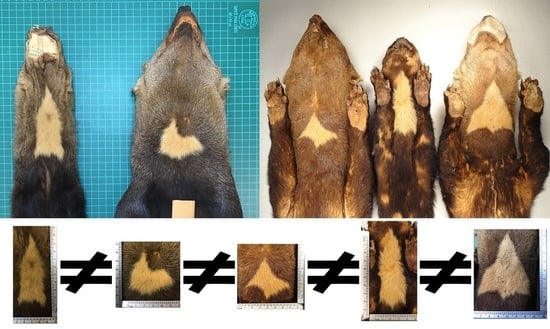
:1. Introduction
2. Materials and Methods
A Wild Population Case Study
3. Results
A Wild Population Case Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

| Location | Name * of the Specimen in the Collection | Code | Length (cm) | Width (cm) | Area (cm2) | Perimeter (cm) | Shape Index | Number in Collection |
|---|---|---|---|---|---|---|---|---|
| Zoo | Tayra barbara ** | A1 | 0.97 | 0.58 | 0.15 | 9.28 | 6.76 | 6856/5516 |
| Argentina | Eira barbara barbara | A2 | 11.5 | 6.08 | 36.3 | 441.98 | 20.70 | 185,325 |
| Brazil | Tayra barbara ** | A3 | 9.6 | 3.9 | 22.5 | 135.46 | 8.06 | 133,958 |
| Brazil | Tayra barbara ** | A4 | 5.25 | 5.6 | 14.03 | 227.41 | 17.13 | 133,952 |
| Brazil | Tayra barbara ** | A5 | 10.3 | 5.5 | 26.13 | 243.13 | 13.42 | 133,955 |
| Paraguay | Tayra barbara galina ** | A7 | 6.02 | 5.91 | 16.09 | 261.88 | 18.42 | 36,507 |
| Honduras | Tayra barbara inserta ** | A8 | 1.59 | 0.74 | 0.53 | 13.64 | 5.29 | 123,271 |
| Honduras | Tayra barbara inserta ** | A9 | 2.61 | 2.46 | 1.92 | 44.66 | 9.09 | 128,127 |
| Brazil | Tayra barbara barbara ** | A13 | 5.08 | 4.77 | 10.53 | 138.99 | 12.09 | 37,479 |
| Bolivia | Eira barbara | A15 | 6.4 | 3.15 | 5.92 | 126.57 | 14.68 | 38,810 |
| Bolivia | Tayra barbara madeirensis ** | A16 | 9.3 | 5.6 | 18.25 | 217.67 | 14.38 | 40,838 |
| Trinidad and Tobago | Tayra barbara trinitatis ** | A17 | 5.94 | 5.1 | 16.19 | 79.25 | 5.56 | 7543–5937 |
| Colombia | Tayra barbara ** | A18 | 5.4 | 5.4 | 14.57 | 95.12 | 7.03 | 134,947 |
| Colombia | Tayra barbara barbara ** | A19 | 10.7 | 6.18 | 32.77 | 303.29 | 14.95 | 37,366 |
| Ecuador | Eira barbara | A24 | 9.31 | 6.99 | 29.93 | 226.44 | 11.68 | 182,953 |
| Brazil | Tayra barbara ** | A26 | 9.9 | 7.3 | 30.38 | 391.45 | 20.04 | 133,953 |
| Venezuela | Tayra barbara barbara ** | A37 | 8.7 | 5.1 | 21.98 | 273.71 | 16.47 | 30,202 |
| Venezuela | Tayra barbara ** | A38 | 12.8 | 5.6 | 25.91 | 266.83 | 14.79 | 16,937 |
| Venezuela | Tayra barbara ** | A39 | 9.9 | 4.06 | 20.62 | 254.03 | 15.79 | 16,938 |
| Mexico | Tayra barbara senex ** | A45 | 5 | 3.36 | 9.05 | 146.26 | 13.72 | 17,254 |
| Colombia | Tayra barbara ** | A46 | 1.47 | 1.07 | 0.69 | 32.19 | 10.93 | 37,799 |
| Colombia | Tayra barbara ** | A47 | 5.25 | 3.8 | 7.75 | 156.42 | 15.85 | 37,800 |
| Costa Rica | Tayra barbara biologiae ** | A48 | 2.71 | 1.3 | 1.3 | 28.47 | 7.05 | 24,444 |
| Ecuador | Tayra barbara senilis ** | A50 | 9.5 | 6.36 | 28.94 | 207.24 | 10.87 | 36,589 |
| Brazil | TAYRA | A54 | 2.86 | 2.42 | 2.53 | 84.56 | 15.00 | 36,230 |
| Colombia | Tayra barbara irara ** | A67 | 8.51 | 8.14 | 23.13 | 206.21 | 12.10 | 14,630 |
| Colombia | Tayra barbara irara ** | A69 | 8.28 | 5.03 | 19.12 | 207.98 | 13.42 | 14,860 |
| Colombia | Tayra barbara irara ** | A70 | 4.34 | 3.91 | 8.47 | 104.11 | 10.09 | 14,861 |
| Colombia | Tayra barbara irara ** | A72 | 4.49 | 2.8 | 4.35 | 74.62 | 10.10 | 15,473 |
| Colombia | Tayra barbara irara ** | A73 | 6.02 | 4.65 | 10.47 | 165.2 | 14.41 | 15,471 |
| Colombia | Tayra barbara irara ** | A77 | 1.59 | 1.74 | 1.2 | 39.57 | 10.19 | 23,485 |
| Colombia | Tayra barbara ** | A85 | 6.96 | 3.55 | 12.66 | 174.13 | 13.81 | 14,224 |
| Colombia | Tayra barbara barbara ** | A87 | 7.47 | 8.37 | 34.32 | 334.7 | 16.12 | 76,747 |
| Colombia | Tayra barbara barbara ** | A88 | 8.5 | 5.28 | 20.81 | 201.66 | 12.47 | 76,748 |
| Peru | Eira barbara | A114 | 5.27 | 4.75 | 11.24 | 132.79 | 11.18 | 230,838 |
| Colombia | Tayra barbara barbara ** | A138 | 4.66 | 1.96 | 3.67 | 74.78 | 11.01 | 32,669 |
| Colombia | Eira barbara biologiae ** | S2 | 6.79 | 4.36 | 8.49 | 78.04 | 7.56 | 281,467 |
| Costa Rica | Tayra barbara biologiae ** | S3 | 7.38 | 5.14 | 17.31 | 156.75 | 10.63 | 8411–38,483 |
| Costa Rica | Tayra barbara biologiae ** | S4 | 3.97 | 2.43 | 3.11 | 58.32 | 9.33 | 11,375 |
| Costa Rica | Tayra barbara biologiae ** | S5 | 7.31 | 4.44 | 9.87 | 169.65 | 15.24 | 12,875 |
| Panama | Tayra barbara biologiae ** | S10 | 1.18 | 0.88 | 0.43 | 27.94 | 12.02 | 171,081 |
| Ecuador | Eira barbara biologiae ** | S11 | 4.51 | 3.17 | 5.06 | 101.3 | 12.71 | 104,547 |
| Peru | Eira barbara peruana | S12 | 5.82 | 6.52 | 12.31 | 177.98 | 14.31 | 149,015 |
| Ecuador | Taya barbara biologiae ** | S14 | 8.05 | 6.2 | 23.29 | 199.95 | 11.69 | 104,546 |
| Guyana | Tayra barbara poliocephala ** | S17 | 11.69 | 8.38 | 45.91 | 307.26 | 12.80 | 172,995 |
| Mexico | Eira barbara senex | S21 | 5.78 | 4.2 | 11.69 | 127.81 | 10.55 | 181,265 |
| Guatemala | Eira barbara senex | S22 | 2.9 | 5.9 | 10.32 | 97.2 | 8.54 | 61,276 |
| Guatemala | Eira barbara senex | S23 | 2.53 | 2.18 | 1.88 | 46.39 | 9.55 | 287,480 |
| Mexico | Eira barbara senex | S24 | 6.43 | 7.25 | 23.24 | 117.37 | 6.87 | 13,070 |
| Mexico | Eira barbara senex | S25 | 4.59 | 4.49 | 8.41 | 170.18 | 16.56 | 100,447 |
| Panama | Tayra barbara ** | S27 | 14.7 | 6.72 | 49.9 | 167.15 | 6.68 | 15,423 |
| Panama | Tayra barbara biologiae ** | S38 | 8.33 | 3.87 | 9.08 | 177.84 | 16.65 | 297,961 |
| Panama | Tayra barbara biologiae ** | S41 | 4.85 | 3.36 | 6.03 | 92.99 | 10.69 | 297,962 |
| Panama | Eira barbara biologiae ** | S42 | 3.12 | 5.99 | 7.46 | 167.86 | 17.34 | 310,671 |
| Panama | Eira barbara biologiae ** | S43 | 2.08 | 1.4 | 1.15 | 29.32 | 7.71 | 310,673 |
| Panama | Eira barbara biologiae ** | S44 | 2.29 | 1.94 | 1.85 | 42.13 | 8.74 | 334,556 |
| Panama | Eira barbara biologiae ** | S50 | 0.96 | 0.69 | 0.17 | 16.88 | 11.55 | 335,772 |
| Guatemala | Eira barbara senex | S68 | 3.55 | 1.52 | 2.32 | 31.92 | 5.91 | 287,482 |
| Venezuela | Eira barbara poliocephala | S69 | 2.07 | 1.19 | 0.99 | 27.95 | 7.93 | 296,625 |
| Guatemala | Eira barbara senex | S70 | 3.03 | 1.76 | 2.41 | 31.35 | 5.70 | 287,481 |
| Mexico | Eira barbara | MX 1 | 5.68 | 5.09 | 12.09 | 144.87 | 11.76 | CNMA-4160 |
| Mexico | Eira barbara senex | MX 2 | 6.82 | 5.4 | 19.48 | 95.58 | 6.11 | CNMA-188 |
| Mexico | Eira barbara | MX 3 | 8.18 | 5 | 19.68 | 226.21 | 14.39 | ZOOMAT-0311-828 |
| Mexico | Eira barbara | MX 4 | 6.61 | 6.22 | 21.23 | 162.84 | 9.97 | ZOOMAT-726 |
| Mexico | Eira barbara | MX 5 | 6.1 | 3.59 | 12.33 | 86.05 | 6.91 | ZOOMAT-0303-123 |
| Mexico | Eira barbara senex | MX 6 | 8.08 | 6.24 | 25.35 | 141.93 | 7.95 | IIB-UV-3451 |
| Mexico | Eira barbara | MX 7 | 7.24 | 5.54 | 18.14 | 215.82 | 14.30 | ECOSUR-5431 |
| Mexico | Eira barbara senex | MX 8 | 9.24 | 5.36 | 20.41 | 147.81 | 9.23 | ECOSUR-5552 |
| Mexico | Eira barbara senex | MX 9 | 2.61 | 3.49 | 4.04 | 41.7 | 5.85 | ECOSUR-1170 |
| Mexico | Eira barbara senex | MX 10 | 3.54 | 2.3 | 2.63 | 59.35 | 10.33 | ECOSUR-2585 |
| Mexico | Eira barbara | MX 11 | 6.42 | 5.81 | 19.09 | 151.47 | 9.78 | CEDESU–UAC-836 |
| Mexico | Eira barbara | MX 12 | 4.91 | 3.45 | 7.51 | 75.66 | 7.79 | CEDESU–UAC-604 |
| Mexico | Eira barbara | MX 13 | 6.81 | 5.06 | 15.59 | 156.94 | 11.22 | CEDESU–UAC–without number |




References
- González-Romero, A. Cinco métodos sencillos para estimar el tamaño de las poblaciones de fauna silvestre. In Manual de Técnicas Para el Estudio de la Fauna; Tessaro, G., González, C.L., Eds.; Universidad Autónoma de Querétaro: Santiago de Querétaro, Mexico, 2011; p. 377. ISBN 07-7740-98-8. [Google Scholar]
- Páez, E.; Lezama, M. Estimaciones de abundancia por marca-recaptura utilizando radiotelemetría. Encuentro 1998, 46, 16–24. [Google Scholar]
- Otis, D.L.; Burnham, K.P.; White, G.C.; Anderson, D.R. Statistical inference from capture data on closed animal populations. Wildl. Monogr. 1978, 62, 3–135. [Google Scholar]
- Borchers, D.L.; Efford, M.G. Spatially Explicit Maximum Likelihood Methods for Capture-Recapture Studies. Biometrics 2008, 64, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, G.L. Guidelines for the Capture, Handling, and Care of Mammals as Approved by the American Society of Mammalogists. J. Mammal. 1998, 79, 1416–1431. [Google Scholar] [CrossRef]
- Testé, E.T.; Denis, D. Bases para la fotoidentificación de las cebras (Equus burchellii) del Parque Zoológico Nacional de Cuba/Basis for the photoidentification of zebras (Equus burchellii) in the National Zoological Garden of Cuba. Rev. Cuba. Cienc. Biol. 2013, 2, 50–68. [Google Scholar]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S. REVIEW: Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Hammond, P.S.; Mizroch, S.A.; Donovan, G.P. Individual Recognition of Cetaceans: Use of Photo-Identification and Other Techniques to Estimate Population Parameters: Report of the International Whaling Commission; Special Issue; International Whaling Commission: Cambridge, UK, 1990; ISBN 906975-23-7. [Google Scholar]
- Harmsen, B.J.; Foster, R.J.; Sanchez, E.; Gutierrez-González, C.E.; Silver, S.C.; Ostro, L.E.T.; Kelly, M.J.; Kay, E.; Quigley, H. Long term monitoring of jaguars in the Cockscomb Basin Wildlife Sanctuary, Belize, Implications for camera trap studies of carnivores. PLoS ONE 2017, 12, e0179505. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.L. Noninvasive Identification of Individual American Badgers by Features of Their Dorsal Head Stripes. West. N. Am. Nat. 2016, 76, 259–261. [Google Scholar] [CrossRef]
- Trujillo, F.; Portocarrero, M.; Gómez, C. Plan de Manejo y Conservación de Especies Amenazadas en la Reserva de Biósfera El Tuparro: Delfines de rio, Manatíes, Nutrias, Jaguares y Tortugas del Género Podocnemis. Proyecto Pijiwi Orinoko (Fundación Omacha-Fundación Horizonte Verde); Forest Conservation Agreement: Bogotá, Colombia, 2008; ISBN 58-97826-5-1.
- Magoun, A.J.; Valkenburg, P.; Lowell, R.E. Habitat Associations and Movement Patterns of Reproductive Female Wolverines (Gulo Gulo Luscus) on the Southeast Alaska Mainland; Wildlife Research Annual Progress Report; Department of Fish and Game: Petersburg, AK, USA, 2008.
- Sirén, A.; Pekins, P.; Abdu, P.; Ducey, M. Identification and Density Estimation of American Martens (Martes americana) Using a Novel Camera-Trap Method. Diversity 2016, 8, 3. [Google Scholar] [CrossRef]
- Presley, S.J. Eira barbara. Mammalian Species. Mamm. Species 2000, 636, 1–6. [Google Scholar] [CrossRef]
- Cabrera, A. Catálogo de los mamíferos de América del Sur. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”. J. Mammal. 1958, 4, 1–307. [Google Scholar]
- Hall, E.R. The Mammals of North America, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1981. [Google Scholar]
- Ruiz-García, M.; Lichilín-Ortiz, N.; Jaramillo, M.F. Molecular phylogenetics of two Neotropical carnivores, Potos flavus (Procyonidae) and Eira barbara (Mustelidae): No clear existence of putative morphological subspecies. Mol. Popul. Genet. Evol. Biol. Biol. Conserv. Neotrop. Carniv. 2013, 32, 37–84. [Google Scholar]
- Camargo, C.C.; Ferrari, S.F. Interactions between tayras (Eira barbara) and red-handed howlers (Alouatta belzebul) in eastern Amazonia. Primates 2007, 48, 147–150. [Google Scholar] [CrossRef] [PubMed]
- López González, C.A.; Lara, A.; Daniel, R. Noteworthy record of the Tayra (Carnivora: Mustelidae: Eira barbara) in the Sierra Gorda biosphere reserve, Querétaro, México. West. N. Am. Nat. 2007, 67, 150–151. [Google Scholar] [CrossRef]
- Ramírez Bravo, O.E. Nuevos registros de tayra (Eira barbara Linnaeus 1758) en Puebla, centro de México. Acta Zool. Mex. 2011, 27, 883–886. [Google Scholar]
- Pérez-Irineo, G.; Santos-Moreno, A. Diversidad de mamíferos terrestres de talla grande y media de una selva subcaducifolia del noreste de Oaxaca, México. Rev. Mex. Biodivers. 2012, 83, 164–169. [Google Scholar]
- González-Maya, J.F.; Zárrate-Charry, D.; Vela-Vargas, I.M.; Jiménez-Alvarado, J.S.; Gómez-Hoyos, D. Activity patterns of Tayra Eira barbara populations from Costa Rica and Colombia: Evidence of seasonal effects Patrones de actividad de poblaciones de la Tayra Eira barbara en Costa Rica y Colombia: Evidencia de efectos estacionales. Rev. Biodivers. Neotrop. 2015, 5, 96–104. [Google Scholar] [CrossRef]
- Reyes-Puig, C.P.; Ríos-Alvear, G.D.; Reyes-Puig, J.P. Notable ampliación del rango altitudinal de Eira barbara Cabeza de Mate (Mammalia: Mustelidae). ACI Av. En Cienc. E Ing. 2015, 7, B098–B102. [Google Scholar]
- García, J.J.M.; García, A.D.M.; Cruz, J.M.C. Registros del tayra (Eira barbara) en el estado de Hidalgo, México. Rev. Mex. Mastozool. Nueva Época 2016, 6, 24–28. [Google Scholar]
- McGarigal, K.; Marks, B.J. Spatial Pattern Analysis Program for Quantifying Landscape Structure; General Technical Report PNW-GTR-351; USA Department of Agriculture, Forest Service Pacific Northwest Research Station: Portland, OR, USA, 1994.
- Vila Subirós, J.; Varga Linde, D.; Llausàs i Pascual, A.; Ribas Palom, A. Conceptos y métodos fundamentales en ecología del paisaje (landscape ecology). Una interpretación desde la geografía. Copyr. Doc. Anàl. Geogr. 2006, 48, 151–166. [Google Scholar]
- Patton, D.R. A diversity index for quantifying habitat “edge”. Wildl. Soc. Bull. (1973–2006) 1975, 3, 171–173. [Google Scholar]
- Kolowski, J.M.; Alonso, A. Density and activity patterns of ocelots (Leopardus pardalis) in northern Peru and the impact of oil exploration activities. Biol. Conserv. 2010, 143, 917–925. [Google Scholar] [CrossRef]
- Avise, J.C.; Ball, R.M. Principles of genealogical concordance in species concepts and biological taxonomy. Oxf. Surv. Evol. Biol. 1990, 7, 45–67. [Google Scholar]
- Tortato, F.R.; Althoff, S.L. Variações na coloração de iraras (Eira barbara Linnaeus, 1758-Carnivora, Mustelidae) da Reserva Biológica Estadual do Sassafrás, Santa Catarina, sul do Brasil. Biota Neotrop. 2007, 7, 365–367. [Google Scholar] [CrossRef]
- Sobroza, T.V.; Gonçalves, A.L.; dos Santos, L.S. Predation attempt and abnormal coat coloration of the tayra (Eira barbara) in the Brazilian Central Amazon. Stud. Neotrop. Fauna Environ. 2016, 51, 231–234. [Google Scholar] [CrossRef]
- Krumbiegel, I. Die Säugetiere der Südamerika-Expeditionen Prof. Dr. Kriegs; Zoologischer Anzeiger: Leipzig, Germany, 1942; Volume 139, pp. 81–96. [Google Scholar]
- Caro, T.M. The adaptive significance of coloration in mammals. BioScience 2005, 55, 125–136. [Google Scholar] [CrossRef]
- Abreu, M.S.L.; Machado, R.; Barbieri, F.; Freitas, N.S.; Oliveira, L.R. Anomalous colour in Neotropical mammals: A review with new records for Didelphis sp. (Didelphidae, Didelphimorphia) and Arctocephalus australis (Otariidae, Carnivora). Braz. J. Biol. 2013, 73, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kettlewell, H.B.D. The Evolution of Melanism: The Study of a Recurring Necessity; Clarendon Press: New York, NY, USA, 1973; ISBN 198573708. [Google Scholar]
- Majerus, M.E.N. Melanism: Evolution in Action; Oxford University Press: New York, NY, USA, 1998; ISBN 198549826. [Google Scholar]
- Elosegi, M.M.; Rubines, J.; Ruíz, A. Hallazgo e identificación molecular de un ejemplar de garduña, Martes foina (Erxleben, 1777) con coloración atípica en Ezkurra (Navarra). Galemys 2006, 18, 23–26. [Google Scholar]
- Chehrsimin, T. Enhanced Methods for Saimaa Ringed Seal Identification. Master’s Thesis, Lappeenranta University of Technology School of Engineering Science, Lappeenranta, Finland, 2015. [Google Scholar]
- Kendall, D.G. The Diffusion of Shape. Adv. Appl. Probab. 1977, 9, 428–430. [Google Scholar] [CrossRef]
- Silver, S.C.; Ostro, L.E.T.; Marsh, L.K.; Maffei, L.; Noss, A.J.; Kelly, M.J.; Wallace, R.B.; Gómez, H.; Ayala, G. The use of camera traps for estimating jaguar Panthera onca abundance and density using capture/recapture analysis. Oryx 2004, 38. [Google Scholar] [CrossRef]
- Royle, J.A.; Chandler, R.B.; Sollmann, R.; Gardner, B. (Eds.) Spatial Capture-Recapture; Elsevier: Amsterdam, The Netherlands, 2014; p. 568. ISBN 978-0-12-405939-9. [Google Scholar]
- Xavier da Silva, M.; Paviolo, A.; Tambosi, L.R.; Pardini, R. Effectiveness of Protected Areas for biodiversity conservation: Mammal occupancy patterns in the Iguaçu National Park, Brazil. J. Nat. Conserv. 2018, 41, 51–62. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Tobler, M.W.; Zúñiga Hartley, A.; Carrillo-Percastegui, S.E.; Powell, G.V.N. Spatiotemporal hierarchical modelling of species richness and occupancy using camera trap data. J. Appl. Ecol. 2015, 52, 413–421. [Google Scholar] [CrossRef]
- Tobler, M.W.; Powell, G.V.N. Estimating jaguar densities with camera traps: Problems with current designs and recommendations for future studies. Biol. Conserv. 2013, 159, 109–118. [Google Scholar] [CrossRef]
- Sollmann, R.; Gardner, B.; Belant, J.L. How Does Spatial Study Design Influence Density Estimates from Spatial Capture-Recapture Models? PLoS ONE 2012, 7, e34575. [Google Scholar] [CrossRef] [PubMed]












| Subspecies | Coloration | Presence of Throat Patch | Observations | Distribution ** | |||
|---|---|---|---|---|---|---|---|
| Body | Legs | Nape | Head | ||||
| barbara 1 | Dull brown | * | * | No distinct gray to brown | Yes, yellowish | Body is lighter than E. b. sinuensis and darker than E. b. senex | Paraguay, part of Brazil, Peru, Bolivia, and Argentina |
| sinuensis 2 | Black | * | Darker brown than the head. | * | May be present | Body is darker than E. b. senex | Panama, part of Costa Rica, Venezuela, Colombia, and Ecuador |
| poliocephala 3 | Dull brown | * | * | Brown | Yes, yellowish | Pelage is similar to that of E. b. barbara but with a darker yellow throat patch and yellow shoulder patches, which sometimes join forming a complete yellow collar | Guyana, French Guyana, Surinam, part of Brazil and Venezuela |
| peruana 4 | Dark chocolate brown | Darker than body | * | * | * | The color of the body is as in E. b. madeirensis, except that limb, are darker than body and tail is black | Part of Peru and Bolivia |
| senex 5 | Dark brown | Grayish white | Yes, yellowish | The grayish white color extends to shoulders fading to a dark yellow | Belize, part of Mexico, Guatemala, and Honduras | ||
| inserta 6 | Black | * | * | Dark brown | No | * | El Salvador, Nicaragua, part of Guatemala, Honduras and Costa Rica |
| madeirensis 7 | Dark chocolate brown | * | Slightly lighter than body | May be present | * | Part of Brazil, Venezuela, Colombia, Peru, and Ecuador | |
| Country | Collections | Number of Specimens | Specimens with Throat Patch | Specimens without Throat Patch | Analyzed Throat Patch | Incomplete Specimens ** |
|---|---|---|---|---|---|---|
| Mexico | Colección Nacional de Mamíferos (CNMA). | 2 | 2 | 0 | 2 | 0 |
| Colección Mastozoológica del Zoológico Miguel Álvarez del Toro (ZOOMAT). | 3 | 3 | 0 | 3 | 0 | |
| Colección de Mamíferos del Instituto de Investigaciones Biológicas de la Universidad Veracruzana (IBB-UV). | 1 | 1 | 0 | 1 | 0 | |
| Laboratorio de Mastozoología de la División Académica de Ciencias Biológicas de la Universidad Juárez Autónoma de Tabasco (DACBIOL-UJAT). | 1 | 1 * | 0 | 0 | 0 | |
| Colección Mastozoológica de El Colegio de la Frontera Sur (ECOSUR). | 4 | 4 | 0 | 4 | 0 | |
| Colección Mastozoológica del Centro de Estudios en Desarrollo Sustentable y Aprovechamiento de la Vida Silvestre de la Universidad Autónoma de Campeche (CEDESU-UAC). | 4 | 3 | 1 | 3 | 0 | |
| United States | The Division of Mammals of the National Museum of Natural History (NMNH), Smithsonian Institution. | 103 | 74 | 14 | 24 | 15 |
| Mammalogy Collection of the American Museum of Natural History (AMNH). | 157 | 133 | 15 | 36 | 8 | |
| Total | 275 | 222 | 30 | 73 | 23 | |
| Country | Specimens with Throat Patch | Specimens without Throat Patch | Incomplete Specimens | Total | Number of Measured Throat Patches |
|---|---|---|---|---|---|
| Mexico | 25 | 5 | 1 | 31 | 17 |
| Guatemala | 7 | 0 | 0 | 7 | 4 |
| Honduras | 2 | 0 | 0 | 2 | 2 |
| El Salvador | 1 | 0 | 0 | 1 | 0 |
| Nicaragua | 3 * | 5 | 1 | 9 | 0 |
| Costa Rica | 9 | 4 | 0 | 13 | 4 |
| Panama | 20 | 9 | 13 | 42 | 8 |
| Colombia | 34 | 0 | 1 | 35 | 15 |
| Venezuela | 17 | 4 | 0 | 21 | 4 |
| Trinidad and Tobago | 1 | 0 | 0 | 1 | 1 |
| Guyana | 13 | 3 | 3 | 19 | 1 |
| Brazil | 27 | 0 | 0 | 27 | 6 |
| Ecuador | 15 | 0 | 2 | 17 | 4 |
| Peru | 27 | 0 | 0 | 27 | 2 |
| Bolivia | 11 | 0 | 0 | 11 | 2 |
| Paraguay | 1 | 0 | 0 | 1 | 1 |
| Argentina | 1 | 0 | 0 | 1 | 1 |
| Zoo ** | 8 | 0 | 2 | 10 | 1 |
| Total | 222 | 30 | 23 | 275 | 73 |
| Country of Origin | Mean Length (cm) | Mean Width (cm) | Mean Area (cm2) | Mean Perimeter (cm) |
|---|---|---|---|---|
| Mexico (n = 17) | 6.12 ± 1.67 | 4.81 ± 1.28 | 14.70 ± 6.82 | 133.40 ± 50.16 |
| Guatemala (n = 4) | 3.00 ± 0.42 | 2.84 ± 2.05 | 4.23 ± 4.06 | 51.71 ± 31.11 |
| Honduras (n = 2) | 2.10 ± 0.72 | 1.60 ± 1.21 | 1.22 ± 0.98 | 29.15 ± 21.93 |
| Costa Rica (n = 4) | 5.34 ± 2.36 | 3.32 ± 1.77 | 7.89 ± 7.27 | 103.29 ± 70.43 |
| Panama (n = 8) | 4.68 ± 4.70 | 3.10 ± 2.30 | 9.50 ± 16.67 | 90.26 ± 70.61 |
| Colombia (n = 15) | 6.02 ± 2.55 | 4.41 ± 2.12 | 13.49 ± 10.59 | 149.86 ± 90.77 |
| Venezuela (n = 4) | 8.36 ± 4.53 | 3.98 ± 1.97 | 17.37 ± 11.15 | 205.63 ± 118.73 |
| Trinidad and Tobago (n = 1) | 5.94 * | 5.10 * | 16.19 * | 79.25 * |
| Guyana (n = 1) | 11.69 * | 8.38 * | 45.91 * | 307.26 * |
| Brazil (n = 6) | 7.16 ± 3.15 | 4.91 ± 1.65 | 17.68 ± 10.48 | 203.50 ± 109.91 |
| Ecuador (n = 4) | 7.84 ± 2.31 | 5.68 ± 1.70 | 21.80 ± 11.54 | 183.73 ± 56.07 |
| Peru (n = 2) | 5.54 ± 0.38 | 5.63 ± 1.25 | 11.75 ± 0.75 | 155.38 ± 31.95 |
| Bolivia (n = 2) | 7.85 ± 2.05 | 4.37 ± 1.73 | 12.08 ± 8.71 | 172.12 ± 64.41 |
| Paraguay (n = 1) | 6.02 * | 5.91 * | 16.09 * | 261.88 * |
| Argentina (n = 1) | 11.50 * | 6.08 * | 36.30 * | 441.98 * |
| Zoo (n = 1) | 0.97 * | 0.58 * | 0.15 * | 9.28 * |
| Observations | Months | Total | |||||
|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | ||
| Number of independent photographic events | 5 | 14 | 8 | 3 | 3 | 2 | 35 |
| Capture events of males | 1 | 10 | 5 | 2 | 1 | 1 | 20 |
| Capture events of females | - | - | - | - | - | - | - |
| Capture events of unknown sex | 5 * | 4 | 5 * | 1 | 2 | 1 | 18 |
| Total capture events of tayras | 6 | 14 | 10 | 3 | 3 | 2 | 38 |
| Number of tayras without visible throat patch ** | 5 | 2 | 4 | 1 | 3 | 2 | 17 |
| Number of tayras with visible throat patch | 1 | 12 | 6 | 2 | - | - | 21 |
| Number of identified tayras | 1 | 12 | 4 | 2 | - | - | 19 |
| Identified individuals | |||||||
| a—unknown sex | - | 1 | - | - | - | - | 1 |
| b—male *** | - | 6 | 1 | 1 | - | - | 8 |
| c—unknown sex | - | - | 1 | - | - | - | 1 |
| d—male *** | - | - | 1 | - | - | - | 1 |
| e—unknown sex | - | 1 | - | - | - | - | 1 |
| f—male *** | - | 2 | 1 | 1 | - | - | 4 |
| g—unknown sex | - | 1 | - | - | - | - | 1 |
| h—unknown sex | 1 | - | - | - | - | - | 1 |
| i—male *** | - | 1 | - | - | - | - | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafañe-Trujillo, Á.J.; López-González, C.A.; Kolowski, J.M. Throat Patch Variation in Tayra (Eira barbara) and the Potential for Individual Identification in the Field. Diversity 2018, 10, 7. https://doi.org/10.3390/d10010007
Villafañe-Trujillo ÁJ, López-González CA, Kolowski JM. Throat Patch Variation in Tayra (Eira barbara) and the Potential for Individual Identification in the Field. Diversity. 2018; 10(1):7. https://doi.org/10.3390/d10010007
Chicago/Turabian StyleVillafañe-Trujillo, Álvaro José, Carlos Alberto López-González, and Joseph M. Kolowski. 2018. "Throat Patch Variation in Tayra (Eira barbara) and the Potential for Individual Identification in the Field" Diversity 10, no. 1: 7. https://doi.org/10.3390/d10010007
APA StyleVillafañe-Trujillo, Á. J., López-González, C. A., & Kolowski, J. M. (2018). Throat Patch Variation in Tayra (Eira barbara) and the Potential for Individual Identification in the Field. Diversity, 10(1), 7. https://doi.org/10.3390/d10010007