Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
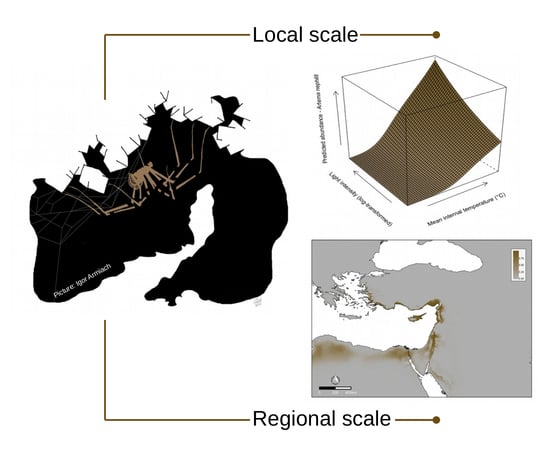
2.2. Local Scale
2.2.1. Field Survey
2.2.2. Local Scale Model
2.3. Regional Scale
2.3.1. Occurrence Data
Environmental Predictors and Calibration Area
2.3.2. Modeling Procedure
3. Results
3.1. Local Scale
3.2. Regional Scale
4. Discussion
4.1. Models Interpretation
4.2. Biogeographic Considerations
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species diversity: Scale matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, R. On the relationship between niche and distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Zobel, M. The relative role of species pools in determining plant species richness: An alternative explanation of species coexistence. Trends Ecol. Evol. 1997, 12, 266–269. [Google Scholar] [CrossRef]
- Gibert, J.; Deharveng, L. Subterranean ecosystems: A truncated functional biodiversity. BioScience 2002, 52, 473–481. [Google Scholar] [CrossRef]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats, 1st ed.; Oxford University Press: Oxford, UK, 2009; p. 272. [Google Scholar]
- Romero, A. Cave Biology: Life in Darkness, 1st ed.; Cambridge University Press: Cambridge, UK, 2009; p. 291. [Google Scholar]
- Culver, D.C.; Trontelj, P.; Zagmajster, M.; Pipan, T. Paving the way for standardized and comparable subterranean biodiversity studies. Subterr. Biol. 2013, 10, 43–50. [Google Scholar] [CrossRef]
- Mammola, S. Finding answers in the dark: Caves as models in ecology fifty years after Poulson and White. Ecography 2018, 42, 1331–1351. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Martins, R.P. Diversity and distribution of spiders associated with bat guano piles in Morrinho cave (Bahia State, Brazil). Divers. Distrib. 1998, 4, 235–241. [Google Scholar]
- Tobin, B.W.; Hutchins, B.T.; Schwartz, B.F. Spatial and temporal changes in invertebrate assemblage structure from the entrance to deep-cave zone of a temperate marble cave. Int. J. Speleol. 2013, 42, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Simões, M.H.; Souza-Silva, M.; Ferreira, R.L. Cave physical attributes influencing the structure of terrestrial invertebrate communities in Neotropics. Subterr. Biol. 2015, 16, 103–121. [Google Scholar] [Green Version]
- Jaffé, R.; Prous, X.; Calux, A.; Gastauer, M.; Nicacio, G.; Zampaulo, R.; Souza-Filho, P.W.M.; Oliveira, G.; Brandi, I.V.; Siqueira, J.O. Conserving relics from ancient underground worlds: Assessing the influence of cave and landscape features on obligate iron cave dwellers from the Eastern Amazon. PeerJ 2018, 6, e4531. [Google Scholar] [CrossRef] [PubMed]
- Christman, M.C.; Culver, D.C. The relationship between cave biodiversity and available habitat. J. Biogeogr. 2001, 28, 367–380. [Google Scholar] [CrossRef]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Cardoso, P. Diversity and community assembly patterns of epigean vs. troglobiont spiders in the Iberian Peninsula. Int. J. Speleol. 2012, 41, 83–94. [Google Scholar] [CrossRef]
- Niemiller, M.L.; Zigler, K.S. Patterns of cave biodiversity and endemism in the Appalachians and Interior Plateau of Tennessee, USA. PLoS ONE 2013, 8, e64177. [Google Scholar] [CrossRef] [PubMed]
- Zagmajster, M.; Eme, D.; Fišer, C.; Galassi, D.; Marmonier, P.; Stoch, F.; Cornu, J.F.; Malard, F. Geographic variation in range size and beta diversity of groundwater crustaceans: Insights from habitats with low thermal seasonality. Glob. Ecol. Biogeogr. 2014, 23, 1135–1145. [Google Scholar] [CrossRef]
- Eme, D.; Zagmajster, M.; Fišer, C.; Galassi, D.; Marmonier, P.; Stoch, F.; Cornu, J.F.; Oberdorff, T.; Malard, F. Multi-causality and spatial non-stationarity in the determinants of groundwater crustacean diversity in Europe. Ecography 2015, 37, 1–10. [Google Scholar] [CrossRef]
- Christman, M.C.; Doctor, D.H.; Niemiller, M.L.; Weary, D.J.; Young, J.A.; Zigler, K.S.; Culver, D.C. Predicting the occurrence of cave-inhabiting fauna based on features of the earth surface environment. PLoS ONE 2016, 11, e0160408. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Sendra, A.; Garay, P.; Reboleira, A.S.P.S. Energy and speleogenesis: Key determinants of terrestrial species richness in caves. Ecol. Evol. 2017, 7, 10207–10215. [Google Scholar] [CrossRef]
- Trajano, E.; Gallão, J.E.; Bichuette, M.E. Spots of high diversity of troglobites in Brazil: The challenge of measuring subterranean diversity. Biodivers. Conserv. 2016, 25, 1805–1828. [Google Scholar] [CrossRef]
- Malard, F.; Boutin, C.; Camacho, A.I.; Ferreira, D.; Michel, G.; Sket, B.; Stoch, F. Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshwater Biol. 2009, 54, 756–776. [Google Scholar] [CrossRef]
- Stoch, F.; Galassi, D.M. Stygobiotic crustacean species richness: A question of numbers, a matter of scale. Hydrobiologia 2010, 653, 217–234. [Google Scholar] [CrossRef]
- Mammola, S.; Leroy, B. Applying species distribution models to caves and other subterranean habitats. Ecography 2018, 41, 1194–1208. [Google Scholar] [CrossRef]
- Galassi, D.M. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453, 227–253. [Google Scholar] [CrossRef]
- Stoch, F.; Fiasca, B.; Di Lorenzo, T.; Porfirio, S.; Petitta, M.; Galassi, D.M. Exploring copepod distribution patterns at three nested spatial scales in a spring system: Habitat partitioning and potential for hydrological bioindication. J. Limnol. 2015, 75. [Google Scholar] [CrossRef]
- Deharveng, L.; Bedos, A. Diversity of terrestrial invertebrates in subterranean habitats. In Cave Ecology. Ecological Studies (Analysis and Synthesis); Moldovan, O., Kováč, L., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 235, pp. 107–172. [Google Scholar]
- Mammola, S.; Isaia, M. Spiders in caves. Proc. R. Soc. Lond. B Biol. Sci. 2017, 284, 20170193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trajano, E.; Carvalho, M.R. Towards a biologically meaningful classification of subterranean organisms: A critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 2017, 22, 1. [Google Scholar] [CrossRef]
- Huber, B.A. Cave-dwelling pholcid spiders (Araneae, Pholcidae): A review. Subterr. Biol. 2018, 26, 1. [Google Scholar] [CrossRef]
- Huber, B.A.; Carvalho, L.S. Filling the gaps: Descriptions of unnamed species included in the latest molecular phylogeny of Pholcidae (Araneae). Zootaxa 2019, 4546, 1–96. [Google Scholar] [CrossRef]
- Aharon, S. Ecology and Taxonomy of the Family Pholcidae in Israel: Species Richness, Geographic Distributions and Taxonomical Revision of the Genus Artema (Pholcidae, Araneae). MSc Thesis, Ben-Gurion University of the Negev, Beersheba, Israel, 2016. Available online: http://nnhc.huji.ac.il/wpcontent/uploads/2017/01/aharonshlomi.pdf. (accessed on 25 July 2019).
- Aharon, S.; Huber, B.A.; Gavish-Regev, E. Daddy-long-leg giants: Revision of the spider genus Artema Walckenaer, 1837 (Araneae, Pholcidae). Eur. J. Taxon. 2017, 376, 1–57. [Google Scholar] [CrossRef]
- Gavish-Regev, E.; Aharon, S.; Armiach, I.; Lubin, Y. Cave survey yields a new spider family record for Israel. Arachnol. Lett. 2016, 51, 39–42. [Google Scholar] [CrossRef]
- Avni, Y. Tectonic and physiographic settings of the Levant. In Quaternary of the Levant: Environments, Climate Change, and Humans, 1st ed.; Enzel, Y., Bar-Yosef, O., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 3–16. [Google Scholar]
- Por, F.D. An outline of the zoogeography of the Levant. Zool. Scr. 1975, 4, 5–20. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Tchernov, E. The Zoogeography of Israel: The Distribution and Abundance at a Zoogeographical Crossroad, 1st ed.; Dr, W., Ed.; Junk Publishers: Dordrecht, The Netherlands, 1988; p. 600. [Google Scholar]
- Frumkin, A. Quaternary evolution of caves and associated Palaeoenvironments of the southern Levant, In Quaternary of the Levant: Environments, Climate Change, and Humans, 1st ed.; Enzel, Y., Bar-Yosef, O., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 135–144. [Google Scholar]
- Bar-Matthews, M.; Ayalon, A.; Vaks, A.; Frumkin, A. Climate and environment reconstructions based on speleothems from the Levant. In Quaternary of the Levant: Environments, Climate Change, and Humans, 1st ed.; Enzel, Y., Bar-Yosef, O., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 151–164. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 1 April 2019).
- Zuur, A.F.; Ieno, E.N.; Elphick, S.C. A protocol for data exploration to avoid common statistical problem. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Carter, J.; Fowles, A.; Angele, C. Monitoring the population of the linyphid spider Porrhomma rosenhaueri (L. Koch, 1872) (Araneae: Linyphiidae) in Lesser Garth Cave, Cardiff, UK. J. Caves Karst Sci. 2010, 37, 3–8. [Google Scholar]
- Mammola, S.; Isaia, M. The ecological niche of a specialized subterranean spider. Invertebr. Biol. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Barry, S.C.; Welsh, A.H. Generalized additive modelling and zero inflated count data. Ecol. Model. 2002, 157, 179–188. [Google Scholar] [CrossRef]
- Zuur, A.F.; Savaliev, A.A.; Ieno, E.N. Zero Inflated Models and Generalized Linear Mixed Models with R, 1st ed.; Highland Statistics Limited: Newburgh, NY, USA, 2012; p. 324. [Google Scholar]
- Vuong, Q.H. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989, 57, 307–333. [Google Scholar] [CrossRef]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression models for count data in R. J. Stat. Softw. 2008, 27, 1–28. [Google Scholar] [CrossRef]
- Jackman, S. PSCL: Classes and Methods for R Developed in the Political Science Computational Laboratory, 1st ed.; Stanford University Press: Stanford, CA, USA, 2012; Available online: http://pscl.stanford.edu/. (accessed on 25 July 2019).
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 1st ed.; Springer-Verlag: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference; 2017; R package version 1.40.0. Available online: https://CRAN.R-project.org/package=MuMIn. (accessed on 25 July 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savaliev, A.A.; Smith, G.M. Mixed Effect Models and Extensions in Ecology with R, 1st ed.; Springer: Berlin, Germany, 2009; p. 574. [Google Scholar]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographical Distributions, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2011; p. 315. [Google Scholar]
- Brandt, L.A.; Benscoterb, A.M.; Harvey, R.; Speroterra, C.; Bucklin, D.; Romañachc, S.S.; Watling, J.I.; Mazzotti, F.J. Comparison of climate envelope models developed using expert-selected variables versus statistical selection. Ecol. Model. 2017, 345, 10–20. [Google Scholar] [CrossRef]
- Fourcade, Y.; Besnard, A.G.; Secondi, J. Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Glob. Ecol. Biogeogr. 2018, 27, 245–256. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Anderson, R.P.; Raza, A. The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: Preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J. Biogeogr. 2010, 37, 1378–1393. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jimenez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberon, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Soberón, J.; Ingenloff, K.; Lira-Noriega, A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Huber, B.A.; Kwapong, P. West African pholcid spiders: An overview, with descriptions of five new species (Araneae, Pholcidae). Eur. J. Taxon. 2013, 59, 1–44. [Google Scholar] [CrossRef]
- Mammola, S.; Isaia, M. Rapid poleward distributional shifts in the European cave-dwelling Meta spiders under the influence of competition dynamics. J. Biogeogr. 2017, 44, 2789–2797. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Mammola, S.; Giachino, P.M.; Piano, E.; Jones, A.; Barberis, M.; Badino, G.; Isaia, M. Ecology and sampling techniques of an understudied subterranean habitat: The Milieu Souterrain Superficiel (MSS). Sci. Nat. 2016, 103, 88. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Badino, G. Underground meteorology. What’s the weather underground? Acta Cars. 2010, 39, 427–448. [Google Scholar] [CrossRef]
- Pipan, T.; López, H.; Oromí, P.; Polak, S.; Culver, D.C. Temperature variation and the presence of troglobionts in terrestrial shallow subterranean habitats. J. Nat. Hist. 2011, 45, 257–273. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Brown, M.C. The physics of caves. In The Science of Speleology, 1st ed.; Ford, T.D., Cullingford, C.H.D., Eds.; Academic Press: London, UK, 1976; pp. 329–358. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling; 2014; R package version 1.0-5. Available online: https://CRAN.R-project.org/package=dismo. (accessed on 25 July 2019).
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for ecological niche models. Methods Ecol. Evol. 2014, 6, 119–120. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods; 2018; R package version 3.0. Available online: https://CRAN.R-proje ct.org/package=ecospat. (accessed on 25 July 2019).
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. 2017. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 19 April 2018).
- Culver, D.C.; Poulson, T.L. Community boundaries: Faunal diversity around a cave entrance. Ann. Spéléol. 1970, 25, 853–860. [Google Scholar]
- Lavoie, K.H.; Helf, K.L.; Poulson, T.L. The biology and ecology of North American cave crickets. J. Cave Karst Stud. 2007, 69, 114–134. [Google Scholar]
- Mammola, S.; Isaia, M. Niche differentiation in Meta bourneti and M. menardi (Araneae, Tetragnathidae) with notes on the life history. Int. J. Speleol. 2014, 43, 343–353. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ 2017, 5, e3169. [Google Scholar] [CrossRef] [PubMed]
- Prous, X.; Ferreira, R.L.; Martins, R.P. Ecotone delimitation: Epigean-hypogean transition in cave ecosystems. Austral. Ecol. 2004, 29, 374–382. [Google Scholar] [CrossRef]
- Prous, X.; Ferreira, R.L.; Jacobi, C.M. The entrance as a complex ecotone in a Neotropical cave. Int. J. Speleol. 2015, 44, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Barr, T.C. Observations on the ecology of caves. Am. Nat. 1967, 101, 475–492. [Google Scholar] [CrossRef]
- Peck, S.B. The effect of cave entrances on the distribution of cave-inhabiting terrestrial arthropods. Int. J. Speleol. 1976, 8, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Sanmartín, I. Dispersal vs. vicariance in the Mediterranean: Historical biogeography of the Palearctic Pachydeminae (Coleoptera, Scarabaeoidea). J. Biogeogr. 2003, 30, 1883–1897. [Google Scholar] [CrossRef]
- Opdam, P. Metapopulation theory and habitat fragmentation: A review of holarctic breeding bird studies. Landsc. Ecol. 1991, 5, 93–106. [Google Scholar] [CrossRef]
- Ceccarelli, F.S.; Opell, B.D.; Haddad, C.R.; Raven, R.J.; Soto, E.M.; Ramirez, M.J. Around the world in eight million years: Historical biogeography and evolution of the spray zone spider Amaurobioides (Araneae: Anyphaenidae). PLoS ONE 2016, 11, e0163740. [Google Scholar] [CrossRef]
- Harrison, S.E.; Harvey, M.S.; Cooper, S.J.B.; Austin, A.D.; Rix, M.G. Across the Indian Ocean: A remarkable example of trans-oceanic dispersal in an austral mygalomorph spider. PLoS ONE 2017, 12, e0180139. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Krosby, M.; Wilsey, C.B.; McGuire, J.L.; Duggan, J.M.; Nogeire, T.M.; Heinrichs, J.A.; Tewksbury, J.J.; Lawler, J.J. Climate-induced range overlap among closely related species. Nat. Clim. Chang. 2015, 5, 883–886. [Google Scholar] [CrossRef]
- Juan, C.; Guzik, M.T.; Jaume, D.; Cooper, S.J.B. Evolution in caves: Darwin’s wrecks of ancient life in the molecular era. Mol. Ecol. 2010, 19, 3865–3880. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.C.; Pipan, T. Climate, abiotic factors, and the evolution of subterranean life. Acta Carsologica 2010, 39, 39–577. [Google Scholar] [CrossRef]
- Howarth, F.G. The evolution of non-relictual tropical troglobites. Int. J. Speleol. 1987, 16, 1. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Ribera, C.; Pavlek, M.; Isaia, M. A synthesis on cave dwelling spiders in Europe. J. Zool. Syst. Evol. Res. 2018, 56, 301–316. [Google Scholar] [CrossRef]
- Cooper, S.J.B.; Hinze, S.; Leys, R.; Watts, C.H.S.; Humphreys, W.F. Islands under the desert: Molecular systematics and evolutionary origins of stygobitic water beetles (Coleoptera: Dytiscidae) from central Western Australia. Invertebr. Sys. 2002, 16, 589–598. [Google Scholar] [CrossRef]
- Leys, R.; Watts, C.H.S.; Cooper, S.J.B.; Humphreys, W. Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini: Bidessini) in the arid zone of Australia. Evolution 2003, 57, 2819–2834. [Google Scholar] [PubMed]
- Aharon, S.; Ballesteros, J.; Crawford, A.; Friske, K.; Gainett, G.; Langford, B.; Santibáñez-López, C.; Ya’aran, S.; Gavish-Regev, E.; Sharma, P. The anatomy of an unstable node: A Levantine relict precipitates phylogenomic dissolution of higher-level relationships of the armored harvestmen (Arachnida: Opiliones: Laniatores). Invertebr. Sys. 2019. [Google Scholar] [CrossRef]



| Geographic Region | Large Caves (>20 m) | Medium Caves (10–20 m) | Small Caves (<10 m) |
|---|---|---|---|
| Northern Israel | Yir’on (cave 1; 33.0679 N, 35.4665 E) (P) Shetula (33.0873 N, 35.3169 E) (P) Berniki (cave 1; 32.7775° N, 35.5401° E) (P) Ezba’ (32.7118° N, 34.9747° E) (P) | Oren (32.7144 N, 34.9749 E) (P) Yonim (32.9236 N, 35.2168 E) (P) Berniki (cave 2; 32.7768 N, 35.5413 E) (P) Susita (32.7793 N, 35.6577 E) (P) | Yir’on (cave 2; 33.0672 N, 35.4672 E) (P) Pelekh (32.9324 N, 35.238 E) (P) Berniki (cave 3; 32.7775 N, 35.5401 E) (P) Horvat Raqqkit (32.7128 N, 35.0123 E) (P) |
| Central Israel and Palestine (West Bank) | Haruva (31.9133 N, 34.9607 E) (P) Bet ‘A’rif (Shoham; 32.0026 N, 34.9642 E) (P) Sali’it (32.2454 N, 35.0456 E) (P) Te’omim (31.7262 N, 35.0217 E) (P) | Perat ‘Inbal (cave 1)* (31.8332 N, 35.3019 E) (PE) Perat Roa’im (cave 2)* (31.8325 N, 35.3130 E) (PE) | Perat (cave 3)* (31.8321 N, 35.3083 E) (PE) Perat Southern Slope* (31.8334 N, 35.3054 E) (PE) Oah (32.0053 N, 34.9722 E) (P) Tinshemet (31.9994 N, 34.9681 E) (P) |
| Southern Israel and Palestine (West Bank) | Ashalim (30.9434 N, 34.7391 E) (PE) Malcham (31.0765 N, 35.3971 E) (E) ‘Ammude ‘Amram (cave 1; 29.6515 N, 34.9336 E) (PE) Zavoa’ Cave (31.2086 N, 35.2311 E) (PE) | Telalim (30.9734 N, 34.7929 E) (PE) Arubotayim (31.1016 N, 35.3900 E) (E) Qumeran* (31.7556 N, 35.459 E) (E) Nahal Ha’Besor (30.9415 N, 34.6961 E) (PE) | ‘Avedat (30.7941 N, 34.7720 E) (PE) Nezirim- Ne’ot HakKikkar (30.9911 N, 35.3465 E) (E) ‘Ammude ‘Amram (cave 2; 29.6518 N, 34.9337 E) (PE) |
| Variable | Source | Ecological Relevance | Permutation Importance (PI) |
|---|---|---|---|
| Solar radiation (kJ m−2 day−1) | [64] | Influences microclimate in superficial caves and shallow subterranean habitats [65]. Proxy for the overall habitat aridity. | 52.1 |
| Mean annual temperature (°C) | [66] | Correspond to the internal temperature of most caves [67]. | 14.8 |
| Temperature annual range (°C) | [66] | Proxies of daily and seasonal thermic excursions in the vicinity of the cave entrance [67] and in other superficial subterranean habitats [65,68]. | — |
| Daily temperature range (°C) | [66] | 31.2 | |
| Annual Precipitation (mm) | [64] | Influences general underground climatic conditions [67]. | — |
| Water vapor pressure (kPa) | [64] | Influence underground microclimatic conditions. Proxy of surface productivity [25]. | 0.3 |
| Wind speed (m s−1) | [64] | Proxy for external energy inputs via anemochoric transportation. Air currents also influence general microclimatic conditions in certain caves [69]. | — |
| Elevation (m) | [66] | Surrogate of topographic heterogeneity and habitat availability [18,19]. It also has a general influence on climatic conditions [67]. | 0.8 |
| Carbonate substrate extent (binary raster) | World Map of Carbonate Rock Outcrops v. 3.0 | Proxy of the general availability of subterranean habitats in carbonate substrates [14,20]. | 0.7 |
| Model Structure | Df | AICc | ∆AICc | wi |
|---|---|---|---|---|
| y~ T_mean + Illuminance | 6 | 172.97 | 0.00 | 0.73 |
| y~ T_mean + Delta_T + Illuminance | 8 | 175.31 | 2.33 | 0.22 |
| y~ T_mean + Delta_T + Humidity + Illuminance | 10 | 178.65 | 5.68 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammola, S.; Aharon, S.; Seifan, M.; Lubin, Y.; Gavish-Regev, E. Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism. Diversity 2019, 11, 119. https://doi.org/10.3390/d11080119
Mammola S, Aharon S, Seifan M, Lubin Y, Gavish-Regev E. Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism. Diversity. 2019; 11(8):119. https://doi.org/10.3390/d11080119
Chicago/Turabian StyleMammola, Stefano, Shlomi Aharon, Merav Seifan, Yael Lubin, and Efrat Gavish-Regev. 2019. "Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism" Diversity 11, no. 8: 119. https://doi.org/10.3390/d11080119
APA StyleMammola, S., Aharon, S., Seifan, M., Lubin, Y., & Gavish-Regev, E. (2019). Exploring the Interplay Between Local and Regional Drivers of Distribution of a Subterranean Organism. Diversity, 11(8), 119. https://doi.org/10.3390/d11080119