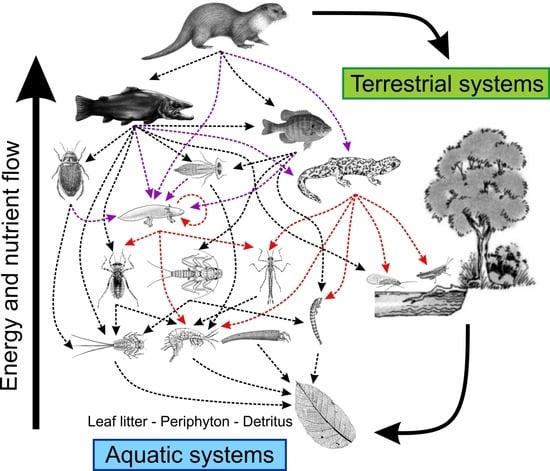
Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways
Abstract
:1. Introduction
2. Salamanders as Predators
2.1. Diet Description
2.2. Top–Down Control
3. Salamanders as Prey
3.1. Consumption of Salamanders in Higher Trophic Levels
3.2. Salamanders as Energy Subsides for Higher Trophic Levels
4. Salamanders as Promoters of Aquatic–Terrestrial Coupling (Lateral Energy Transfers)
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ivlev, V.S. Transformation of energy by aquatic animals. Int. Rev. Ges. Hydrobiol. Hydrogr. 1939, 38, 449–458. [Google Scholar] [CrossRef]
- Lindeman, R. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–418. [Google Scholar] [CrossRef]
- Burton, T.M.; Likens, G.E. Energy flow and nutrient cycling in salamander populations in the Hubbard Brook experimental forest, New Hampshire. Ecology 1975, 56, 1068–1080. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O.; Hladyz, S.; Kitching, R.L.; Martinez, N.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B.; et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.J.; Cavanagh, R.D.; Drinkwater, K.F.; Grant, S.M.; Heymans, J.J.; Hofmann, E.E.; Hunt, G.L.; Johnston, N.M. Understanding the structure and functioning of polar pelagic ecosystems to predict the impacts of change. Proc. R. Soc. B 2016, 283, 20161646. [Google Scholar] [CrossRef] [Green Version]
- Nakano, S.; Miyasaka, H.; Kuhara, N. Terrestrial aquatic linkages: Riparian arthropod inputs alter trophic cascades in a stream food web. Ecology 1999, 80, 2435–2441. [Google Scholar]
- Davic, R.D.; Welsh, H.H.J. On the ecological roles of salamanders. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Power, M.E.; Holomuzki, J.R.; Lowe, R.L. Food webs in Mediterranean rivers. Hydrobiologia 2013, 719, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.D.; Castillo, M.M. Stream Ecology Structure and Function of Running Waters, 2nd ed.; Chapman and Hall: New York, NY, USA, 2007. [Google Scholar]
- Holomuzki, J.R.; Collins, J.P.; Brunkow, P.E. Trophic control of fishless ponds by tiger salamander larvae. Oikos 1994, 71, 55–64. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Effects of Red-Backed Salamanders on Ecosystem Functions. PLoS ONE 2014, 9, e86854. [Google Scholar] [CrossRef] [Green Version]
- Nery, T.; Schmera, D. The effects of top-down and bottom-up controls on macroinvertebrate assemblages in headwater streams. Hydrobiologia 2016, 763, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Semlitsch, R.D.; O’Donnell, K.M.; Thompson, F.R. Abundance, biomass production, nutrient content, and the possible role of terrestrial salamanders in Missouri Ozark forest ecosystems. Can. J. Zool. 2014, 92, 997–1004. [Google Scholar] [CrossRef]
- Fasola, M.; Canova, L. Feeding habits of Triturus vulgaris, T. cristatus and T. alpestris (Amphibia, Urodela) in the northern Apennines (Italy). Boll Zool. 1992, 59, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Montori, A. Alimentación de las larvas de tritón pirenaico, Euproctus asper, en el prepirineo de la Cerdaña, España. Amphibia–Reptilia 1992, 13, 157–167. [Google Scholar] [CrossRef]
- Vignoli, L.; Bombi, P.; D’Amen, M.; Bologna, M.A. Seasonal variation in the trophic niche of a heterochronic population of Triturus alpestris apuanus from the south-western Alps. Herpetol. J. 2007, 17, 183–191. [Google Scholar]
- Romano, A.; Salvidio, S.; Palozzi, R.; Sbordoni, V. Diet of the newt, Triturus carnifex (Laurenti, 1768), in the flooded karst sinkhole Pozzo del Merro, central Italy. J. Caves Karst. Stud. 2012, 74, 271–277. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Montori, A.; Llorente, G.A. Ontogenetic dietary shifts and food resource partitioning in a stream-dwelling Urodela community: Mechanisms to allow coexistence across seasons. Russ. J. Herpetol. 2019, 26, 135–149. [Google Scholar] [CrossRef]
- Joly, P.; Giacoma, C. Limitation of similarity and feeding habits in three syntopic species of newts (Triturus, Amphibia). Ecography 1992, 15, 401–411. [Google Scholar] [CrossRef]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Costa, A.; Romano, A. Trophic specialization at the individual level in a terrestrial generalist salamander. Can. J. Zool. 2015, 93, 79–83. [Google Scholar] [CrossRef]
- Keitzer, S.C.; Goforthm, R.R. Spatial and seasonal variation in the ecological significance of nutrient recycling by larval salamanders in Appalachian headwater streams. Freshw. Sci. 2013, 32, 1136–1147. [Google Scholar] [CrossRef]
- Best, M.L.; Welsh, H.H. The trophic role of a forest salamander: Impacts on invertebrates, leaf litter retention, and the humification process. Ecosphere 2014, 5, 16. [Google Scholar] [CrossRef]
- Regester, K.J.; Whiles, M.R.; Lips, K.R. Variation in the trophic basis of production and energy flow associated with emergence of larval salamander assemblages from forest ponds. Freshwat. Biol. 2008, 53, 1754–1767. [Google Scholar] [CrossRef]
- Braña, F.; de la Hoz, M.; Lastra, C. Alimentación y relaciones tróficas entre las larvas de Triturus marmoratus, T. alpestris y T. helveticus (Amphibia, Caudata). Doñana Acta Vert. 1986, 13, 21–33. [Google Scholar]
- Rosa, G.; Costa, A.; Salvidio, S. Trophic strategies of two newt populations living in contrasting habitats on a Mediterranean island. Ethol. Ecol. Evol. 2019. [Google Scholar] [CrossRef]
- Parker, M.S. Predation by Pacific giant salamander larvae on juvenile steelhead trout. Northwest Nat. 1993, 74, 77–81. [Google Scholar] [CrossRef]
- Montaña, C.G.; Ceneviva-Bastos, M.; Schalk, C.M. New vertebrate prey for the aquatic salamander Amphiuma means (Caudata: Amphiumidae). Herpetol. Notes 2014, 7, 755–756. [Google Scholar]
- Park, S.-R.; Jeong, J.-Y.; Park, D. Cannibalism in the Korean salamander (Hynobius leechii: Hynobiidae, caudata, amphibia) larvae. Integr. Biosci. 2005, 9, 13–18. [Google Scholar] [CrossRef]
- Jefferson, D.M.; Ferrari, M.C.O.; Mathis, A.; Hobson, K.A.; Britzke, E.R.; Crane, A.L.; Blaustein, A.R.; Chivers, D.P. Shifty salamanders: Transient trophic polymorphism and cannibalism within natural populations of larval ambystomatid salamanders. Front. Zool. 2014, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, F.; Perez-Mellado, V.; Gil, M.J.; Lizana, M. Food habits and trophic availability in the high mountain population of the spotted salamander from Spain (Salamandra salamandra almanzoris) (Caudata: Salamandridae). Folia Zool. 1990, 89, 841–853. [Google Scholar]
- Jaeger, R.G. Diet diversity and clutch size of aquatic and terrestrial salamanders. Oecologia 1981, 48, 190–193. [Google Scholar] [CrossRef]
- Salvidio, S.; Crovetto, F.; Costa, A. Individual trophic specialisation in the Alpine newt increases with increasing resource diversity. Ann. Zool. Fenn. 2019, 56, 17–24. [Google Scholar] [CrossRef]
- Ranta, E.; Tjossem, S.F.; Leikola, N. Female-male activity and zooplankton foraging by the smooth newt (Triturus vulgaris). Ann. Zool. Fennici. 1987, 24, 79–88. [Google Scholar]
- Denoël, M.; Joly, P. Size-related predation reduces intramorph competition in paedomorphic alpine newts. Can. J. Zool. 2001, 79, 943–948. [Google Scholar] [CrossRef]
- Maerz, J.C.; Myers, E.M.; Adams, D.C. Trophic polymorphism in a terrestrial salamander. Evol. Ecol. Res. 2006, 8, 23–35. [Google Scholar]
- Sánchez-Hernández, J. Disentangling prey-handling efficiency of larval newts through multivariate prey trait analysis. J. Nat. Hist. 2014, 48, 1957–1969. [Google Scholar] [CrossRef]
- Emlen, J.M. The role of time and energy in food preference. Am. Nat. 1966, 100, 611–617. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Pyke, G.H.; Pulliam, H.R.; Charnov, E.L. Optimal foraging: A selective review of theory and tests. Q. Rev. Biol. 1977, 52, 137–154. [Google Scholar] [CrossRef] [Green Version]
- Cicort-Lucaciu, A.-Ş.; Ardeleanu, A.; Cupşa, D.; Naghi, N. The trophic spectrum of a Triturus cristatus (Laurentus 1768) population from Plopiş Mountains area (Bihor County, Romania). North-West J. Zool. 2005, 1, 31–39. [Google Scholar]
- Crump, M.L. Opportunistic cannibalism by amphibian larvae in temporary aquatic environments. Am. Nat. 1983, 121, 281–287. [Google Scholar] [CrossRef]
- Crossland, M.R. Predation by tadpoles on toxic toad eggs: The effect of tadpole size on predation success and tadpole survival. J. Herpetol. 1998, 32, 443–446. [Google Scholar] [CrossRef]
- de Crespin de Billy, V.; Usseglio-Polatera, P. Traits of brown trout prey in relation to habitat characteristics and benthic invertebrate communities. J. Fish Biol. 2002, 60, 687–714. [Google Scholar] [CrossRef]
- de Crespin de Billy, V.; Dumont, B.; Lagarrigue, T.; Baran, P.; Statzner, B. Invertebrate accessibility and vulnerability in the analysis of brown trout (Salmo trutta L.) summer habitat suitability. River Res. Appl. 2002, 18, 533–553. [Google Scholar] [CrossRef]
- Sánhez-Hernández, J.; Vieira-Lanero, R.; Servia, M.J.; Cobo, F. Feeding habits of four sympatric fish species in the Iberian Peninsula: Keys to understanding coexistence using prey traits. Hydrobiologia 2011, 667, 119–132. [Google Scholar] [CrossRef]
- Ranta, E.; Nuutinen, V. Foraging by the smooth newt (Triturus vulgaris) on zooplankton: Functional response and diet choice. J. Anim. Ecol. 1985, 54, 275–293. [Google Scholar] [CrossRef]
- Ranta, E.; Saloheimo, M.; Nuutinen, V. Orientation of the smooth newt (Triturus vulgaris) foraging on zooplankton. Ann Zool Fennici. 1986, 23, 281–287. [Google Scholar]
- Heiss, E.; Natchev, N.; Gumpenberger, M.; Weissenbacher, A.; Van Wassenbergh, S. Biomechanics and hydrodynamics of prey capture in the Chinese giant salamander reveal a high-performance jaw-powered suction feeding mechanism. J. R. Soc. Interface 2013, 10, 20121028. [Google Scholar] [CrossRef] [Green Version]
- Kopecký, O.; Vojar, J.; Šusta, F.; Rehák, I. Composing and scaling of male and female alpine newt (Mesotriton alpestris) prey, with related site and seasonal effect. Ann. Zool. Fenn. 2012, 9, 231–239. [Google Scholar] [CrossRef]
- Farasat, H.; Sharifi, M. Food habit of the endangered yellow-spotted newt Neurergus microspilotus (Caudata, Salamandridae) in Kavat Stream, western Iran. Zool. Stud. 2014, 53, 61. [Google Scholar] [CrossRef] [Green Version]
- Falke, L.P.; Henderson, J.S.; Novak, M.; Preston, D.L. Temporal shifts in intraspecific and interspecific diet variation: Effects of predator body size and identity across seasons in a stream community. bioRxiv 2018. [Google Scholar] [CrossRef]
- Lunghi, E.; Cianferoni, F.; Ceccolini, F.; Veith, M.; Manenti, R.; Mancinelli, G.; Corti, C.; Ficetola, G.F. What shapes the trophic niche of European plethodontid salamanders? PLoS ONE 2018, 13, e0205672. [Google Scholar] [CrossRef] [PubMed]
- Clavero, M.; Prenda, J.; Delibes, M. Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J. Biogeogr. 2003, 30, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Hernández, J.; Finstad, A.G.; Arnekleiv, J.V.; Kjærstad, G.; Amundsen, P.-A. Drivers of diet patterns in a globally distributed freshwater fish species. Can. J. Fish. Aquat. Sci. 2019, 76, 1263–1274. [Google Scholar] [CrossRef]
- Holomuzki, J.R.; Collins, J.P. Trophic dynamics of a top predator, Ambystoma tigrinum nebulosum (Caudata: Ambystomatidae), in a lentic community. Copeia 1987, 1987, 949–957. [Google Scholar] [CrossRef]
- Parker, M.S. Feeding Ecology of Stream-Dwelling Pacific Giant Salamander Larvae (Dicamptodon tenebrosus). Copeia 1994, 1994, 705–718. [Google Scholar] [CrossRef]
- Wissinger, S.A.; Whiteman, H.H.; Sparks, G.B.; Rouse, G.L.; Brown, W.S. Foraging trade-offs along a predator-permanence gradient in subalpine wetlands. Ecology 1999, 80, 2102–2116. [Google Scholar]
- Sánchez-Hernández, J.; Cobo, F.; Amundsen, P.-A. Food Web Topology in High Mountain Lakes. PLoS ONE 2015, 10, e0143016. [Google Scholar] [CrossRef] [Green Version]
- Urban, M.C. Evolution mediates the effects of apex predation on aquatic food webs. Proc. R. Soc. B 2013, 280, 20130859. [Google Scholar] [CrossRef]
- Atlas, W.I.; Palen, W.J. Prey Vulnerability Limits Top-Down Control and Alters Reciprocal Feedbacks in a Subsidized Model Food Web. PLoS ONE 2014, 9, e85830. [Google Scholar] [CrossRef] [Green Version]
- Hickerson, C.A.M.; Anthony, C.D.; Walton, B.M. Eastern Red-backed Salamanders regulate top-down effects in a temperate forest-floor community. Herpetologica 2017, 73, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.L.; Rowland, F.E.; Semlitsch, R.D. Variation in phenology and density differentially affects predator–prey interactions between salamanders. Oecologia 2017, 185, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Rowland, F.E.; Rawlings, M.B.; Semlitsch, R.D. Joint effects of resources and amphibians on pond ecosystems. Oecologia 2017, 183, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Reice, S.R.; Edwards, R.L. The effect of vertebrate predation on lotic macroinvertebrate communities in Quebec, Canada. Can. J. Zool. 1986, 64, 1930–1936. [Google Scholar] [CrossRef]
- Atlas, W.I.; Palen, W.J.; Courcelles, D.M.; Munshaw, R.G.; Monteith, Z.L. Dependence of stream predators on terrestrial prey fluxes: Food web responses to subsidized predation. Ecosphere 2013, 4, 69. [Google Scholar] [CrossRef]
- Reinhardt, T.; Brauns, M.; Steinfartz, S.; Weitere, M. Effects of salamander larvae on food webs in highly subsidised ephemeral ponds. Hydrobiologia 2017, 799, 37–48. [Google Scholar] [CrossRef]
- Preston, D.L.; Johnson, P.T.J. Importance of Native Amphibians in the Diet and Distribution of the Aquatic Gartersnake (Thamnophis atratus) in the San Francisco Bay Area of California. J. Herpetol. 2012, 46, 221–227. [Google Scholar] [CrossRef]
- Jobe, K.L.; Montaña, C.G.; Schalk, C.M. Emergent patterns between salamander prey and their predators. Food Webs 2019. [Google Scholar] [CrossRef]
- Brandon, R.A.; Huheey, J.E. Diurnal Activity, Avian Predation, and the Question of Warning Coloration and Cryptic Coloration in Salamanders. Herpetologica 1975, 31, 252–255. [Google Scholar]
- Brodie, E.D.; Ridenhour, B.J.; Brodie, E.D. The evolutionary response of predators to dangerous prey: Hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 2002, 56, 2067–2082. [Google Scholar] [CrossRef]
- Brodie, E.D.; Feldman, C.R.; Hanifin, C.T.; Motychak, J.E.; Mulcahy, D.G.; Williams, B.L.; Brodie, E.D. Parallel Arms Races between Garter Snakes and Newts Involving Tetrodotoxin as the Phenotypic Interface of Coevolution. J. Chem. Ecol. 2005, 31, 343–356. [Google Scholar] [CrossRef]
- Stokes, A.N.; Ray, A.M.; Buktenica, M.W.; Gall, B.G.; Paulson, E.; Paulson, D.; French, S.S.; Brodie, E.D.; Brodie, E.D. Otter Predation on Taricha granulosa and Variation in Tetrodotoxin Levels with Elevation. Northwest Nat. 2015, 96, 13–21. [Google Scholar] [CrossRef]
- Bringsøe, H.; Nørgaard, J. Predation of Triturus cristatus (Caudata: Salamandridae) by the Eurasian otter, Lutra lutra (Carnivora: Mustelidae). Herpetol. Notes 2018, 11, 279–280. [Google Scholar]
- Gregory, P.T.; Isaac, L.A. Food Habits of the Grass Snake in Southeastern England: Is Natrix natrix a Generalist Predator? J. Herpetol. 2004, 38, 88–95. [Google Scholar] [CrossRef]
- Escoriza, D.; Hassine, J.B. First case of predation in Pleurodeles poireti (Gervais, 1835). Bol. Asoc. Herpetol. Esp. 2017, 28, 19–20. [Google Scholar]
- Willson, J.D.; Winne, C.T. Evaluating the functional importance of secretive species: A case study of aquatic snake predators in isolated wetlands. J. Zool. 2015, 298, 266–273. [Google Scholar] [CrossRef]
- Willson, J.D.; Hopkins, W.A. Prey morphology constrains the feeding ecology of an aquatic generalist predator. Ecology 2011, 92, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Velo-Antón, G.; Cordero-Rivera, A. Predation by invasive mammals on an insular viviparous population of Salamandra salamandra. Herpetol. Notes 2011, 4, 299–301. [Google Scholar]
- Parry, G.S.; Yonow, N.; Forman, D. Predation of newts (Salamandridae, Pleurodelinae) by Eurasian otters Lutra lutra (Linnaeus). Herpetol. Bull. 2015, 132, 9–14. [Google Scholar]
- Smiroldo, G.; Villa, A.; Tremolada, P.; Gariano, P.; Balestrieri, A.; Delfino, M. Amphibians in Eurasian otter Lutra lutra diet: Osteological identification unveils hidden prey richness and male-biased predation on anurans. Mammal Rev. 2019. [Google Scholar] [CrossRef]
- Clavero, M.; Prenda, J.; Delibes, M. Amphibian and reptile consumption by otters (Lutra lutra) in a coastal area in Southern Iberian Peninsula. Herpetol. J. 2005, 15, 125–131. [Google Scholar]
- Novais, A.; Sedlmayr, A.; Moreira-Santos, M.; Goncalves, F.; Ribeiro, R. Diet of the otter Lutra lutra in an almost pristine Portuguese river: Seasonality and analysis of fish prey through scale and vertebrae keys and length relationships. Mammalia 2010, 74, 71–81. [Google Scholar] [CrossRef]
- Cogălniceanu, D.; Márquez, R.; Beltrán, J.F. Impact of otter (Lutra lutra) predation on amphibians in temporary ponds in Southern Spain. Acta Herpetol. 2010, 5, 217–222. [Google Scholar]
- Hanifin, C.T. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 2010, 8, 577–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiroldo, G.; Gariano, P.; Balestrieri, A.; Manenti, R.; Pini, E.; Tremolada, P. Predation on Amphibians May Enhance Eurasian Otter Recovery in Southern Italy. Zool. Sci. 2019, 36, 273–283. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Interactions between fish and salamander larvae. Oecologia 1987, 72, 481–486. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Allotopic Distribution of Two Salamanders: Effects of Fish Predation and Competitive Interactions. Copeia 1988, 1988, 290–298. [Google Scholar] [CrossRef]
- Domínguez, J.; Pena, J.C. Alimentación del lucio Esox lucius en un área de reciente colonización (cuenca del Esla, noroeste de España). Variaciones en función de la talla. Ecologia 2001, 15, 293–308. [Google Scholar]
- Knapp, R.A. Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biol. Conserv. 2005, 121, 265–279. [Google Scholar] [CrossRef]
- Kats, L.B.; Ferrer, R.P. Alien predators and amphibian declines: Review of two decades of science and the transition to conservation. Divers. Distrib. 2003, 9, 99–110. [Google Scholar] [CrossRef]
- Orizaola, G.; Braña, F. Effect of salmonid introduction and other environmental characteristics on amphibian distribution and abundance in mountain lakes of northern Spain. Anim. Conservat. 2006, 9, 171–178. [Google Scholar] [CrossRef]
- Tiberti, R.; Bogliani, G.; Brighenti, S.; Iacobuzio, R.; Liautaud, K.; Rolla, M.; von Hardenberg, A.; Bassano, B. Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol. Invasions 2019, 21, 875–894. [Google Scholar] [CrossRef]
- Martínez-Solano, I.; Barbadillo, J.; Lapeña, M. Effect of introduced fish on amphibian species richness and densities at a montane assemblage in the Sierra de Neila, Spain. Herpetol. J. 2003, 13, 167–173. [Google Scholar]
- Domínguez, J.; Pena, J.C. Spatio-temporal variation in the diet of northern pike (Esox lucius) in a colonised area (Esla basin, NW Spain). Limnetica 2000, 19, 1–20. [Google Scholar]
- Cobo, F.; Sánchez-Hernández, J.; Vieira-Lanero, R.; Servia, M.J. Organic pollution induces domestication-like characteristics in feral populations of brown trout (Salmo trutta). Hydrobiologia 2013, 705, 119–134. [Google Scholar] [CrossRef]
- Adrián, M.I.; Delibes, M. Food habits of the otter (Lutra lutra) in two habitats of the Doñana National Park, SW Spain. J. Zool. 1987, 212, 399–406. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Gustafson, M.P. Intraguild predation among larval plethodontid salamanders: A field experiment in artificial stream pools. Oecologia 1993, 96, 271–275. [Google Scholar] [CrossRef]
- Yurewicz, K.L. A growth/mortality trade-off in larval salamanders and the coexistence of intraguild predators and prey. Oecologia 2004, 138, 102–111. [Google Scholar] [CrossRef]
- Anderson, T.L.; Semlitsch, R.D. Top predators and habitat complexity alter an intraguild predation module in pond communities. J. Anim. Ecol. 2016, 85, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.L.; Linares, C.; Dodson, K.N.; Semlitsch, R.D. Variability in functional response curves among larval salamanders: Comparisons across species and size classes. Can. J. Zool. 2016, 94, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Resetarits, W.J., Jr. Ecological interactions among predators in experimental stream communities. Ecology 1991, 72, 1782–1793. [Google Scholar] [CrossRef]
- Nyman, S.; Wilkinson, R.F.; Hutcherson, J.E. Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum). J. Herpet. 1993, 27, 78–84. [Google Scholar] [CrossRef]
- Wildy, E.L.; Chivers, D.P.; Kiesecker, J.M.; Blaustein, A.R. The effects of food level and conspecific density on biting and cannibalism in larval long-toed salamanders, Ambystoma macrodactylum. Oecologia 2001, 128, 202–209. [Google Scholar] [CrossRef]
- Polis, G.A. The Evolution and Dynamics of Intraspecific Predation. Annu. Rev. Ecol. Evol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Regester, K.J.; Lips, K.R.; Whiles, M.R. Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in southern Illinois. Oecologia 2006, 147, 303–314. [Google Scholar] [CrossRef]
- Milanovich, J.R.; Maerz, J.C.; Rosemond, A.D. Stoichiometry and estimates of nutrient standing stocks of larval salamanders in Appalachian headwater streams. Freshw. Biol. 2015, 60, 1340–1353. [Google Scholar] [CrossRef]
- Prater, C.; Scott, D.E.; Lance, S.L.; Nunziata, S.O.; Sherman, R.; Tomczyk, N.; Capps, K.A.; Jeyasingh, P.D. Understanding variation in salamander ionomes: A nutrient balance approach. Freshw. Biol. 2019, 64, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Munshaw, R.G.; Palen, W.J.; Courcelles, D.M.; Finlay, J.C. Predator-Driven Nutrient Recycling in California Stream Ecosystems. PLoS ONE 2013, 8, e58542. [Google Scholar] [CrossRef] [Green Version]
- Milanovich, J.R.; Hopton, M.E. Stoichiometry of Excreta and Excretion Rates of a Stream-dwelling Plethodontid Salamander. Copeia 2016, 104, 26–34. [Google Scholar] [CrossRef]
- Efford, I.E. Energy transfer in Marion Lake, British Columbia; with particular reference to fish feeding. Verh. Int. Ver. Limnol. 1969, 17, 104–108. [Google Scholar] [CrossRef]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 1997, 277, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Pough, F.H. Amphibians and reptiles as low energy systems. In Behavioral Energetics; The Cost of Survival in Vertebrates; Aspey, W.P., Lustick, S.I., Eds.; Ohio State University Press: Columbus, OH, USA, 1983; pp. 141–188. [Google Scholar]
- Yang, L.H.; Bastow, J.L.; Spence, K.O.; Wright, A.N. What can we learn from resource pulses? Ecology 2008, 89, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Whiles, M.R.; Jensen, J.B.; Palis, J.G. Diets of larval flatwoods salamanders, Ambystoma cingulatum, from Florida and South Carolina. J. Herpetol. 2004, 38, 208–214. [Google Scholar] [CrossRef]
- Denoël, M. Terrestrial versus aquatic foraging in juvenile Alpine newts (Triturus alpestris). Ecoscience 2004, 11, 404–409. [Google Scholar] [CrossRef]
- Pagacz, S.; Witczuk, J. Intensive Exploitation of Amphibians by Eurasian Otter (Lutra lutra) in the Wołosaty Stream, Southeastern Poland. Ann. Zool. Fenn. 2010, 47, 403–410. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Sánchez-Hernández, J. Feeding studies take guts—Critical review and recommendations of methods for stomach contents analysis in fish. J. Fish Biol. 2019, 95, 1364–1373. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.R.; Rettig, J.E.; Mittelbach, G.G.; Valiulis, J.L.; Schaack, S.R. The effects of fish on assemblages of amphibians in ponds: A field experiment. Freshw. Biol. 1999, 41, 829–837. [Google Scholar] [CrossRef]
- Nakano, S.; Murakami, M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl. Acad. Sci. USA 2001, 98, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Greene, B.T.; Lowe, W.H.; Likens, G.E. Forest succession and prey availability influence the strength and scale of terrestrial-aquatic linkages in a headwater salamander system. Freshw. Biol. 2008, 53, 2234–2243. [Google Scholar] [CrossRef]
- Montori, A. Alimentación de los adultos de Euproctus asper (Dugès, 1852) en la montaña media del Prepirineo catalán (España). Rev. Esp. Herpetol. 1991, 5, 23–36. [Google Scholar]
- Semlitsch, R.D. Differentiating migration and dispersal processes for pond-breeding amphibians. J. Wildl. Manag. 2008, 72, 260–267. [Google Scholar] [CrossRef]
- Reinhardt, T.; Steinfartz, S.; Paetzold, A.; Weitere, M. Linking the evolution of habitat choice to ecosystem functioning: Direct and indirect effects of pond-reproducing fire salamanders on aquatic-terrestrial subsidies. Oecologia 2013, 173, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.L.; Sears, B.R.; Wooten, J.A.; Camp, C.D.; Falk, A.; O’Quin, K.; Pauley, T.K. Evolution of dentition in salamanders: Relative roles of phylogeny and diet. Biol. J. Linn. Soc. 2016, 119, 960–973. [Google Scholar] [CrossRef]
- Rabosky, D.L.; Chang, J.; Title, P.O.; Cowman, P.F.; Sallan, L.; Friedman, M.; Kaschner, K.; Garilao, C.; Near, T.J.; Coll, M.; et al. An inverse latitudinal gradient in speciation rate for marine fishes. Nature 2018, 559, 392–395. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, J. Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways. Diversity 2020, 12, 32. https://doi.org/10.3390/d12010032
Sánchez-Hernández J. Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways. Diversity. 2020; 12(1):32. https://doi.org/10.3390/d12010032
Chicago/Turabian StyleSánchez-Hernández, Javier. 2020. "Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways" Diversity 12, no. 1: 32. https://doi.org/10.3390/d12010032
APA StyleSánchez-Hernández, J. (2020). Reciprocal Role of Salamanders in Aquatic Energy Flow Pathways. Diversity, 12(1), 32. https://doi.org/10.3390/d12010032