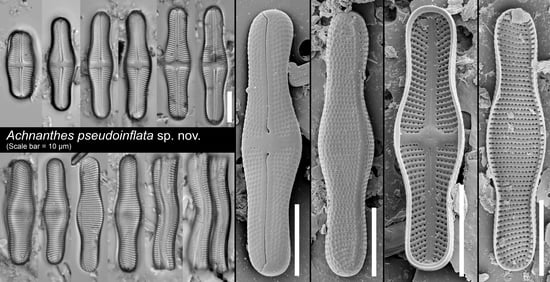
Achnanthes Bory Sensu Stricto (Bacillariophyta) from Terrestrial Habitats of Rio de Janeiro (Brazil), with Description of Achnanthes pseudoinflata sp. nov.
Abstract
:1. Introduction
2. Materials and Methods
Study Area
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Saint-Vincent, B. Achnanthe. Achnanthes. (V. pl. de ce Dict., Arthrodiées, f. 2). In Dictionnaire Classique d’Histoire Naturelle; Audouin, I., Bourdon, A., Brongniart, D., de Fér, D., Desmoulins, A., Drapiez, E., de Saint-Hilaire, F.G., De Jussieu, A., Kunth, G., de Lafosse, L., et al., Eds.; Ouvrage dirigé par ce dernier collaborateur, et dans lequel on a ajouté, pour le porter au niveau de la science, un grand nombre de mots qui n’avaient pu faire partie de la plupart des Dictionnaires antérieurs; Baudoin Frères: Pairs, France, 1822. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms. Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; 747p. [Google Scholar]
- Toyoda, K.; Cox, E.J.; Sims, P.A.; Williams, D.M. The typification of Achnanthes Bory based on Echinella stipitata Lyngbye, with an account of the morphology and fine structure of Lyngbye’s species. Diatom Res. 2005, 20, 375–386. [Google Scholar] [CrossRef]
- Cox, E.J. Achnanthes sensu stricto belongs with genera of the Mastogloiales rather than with other monoraphid diatoms (Bacillariophyta). Eur. J. Phycol. 2006, 41, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.C.; Cocquyt, C. Diatoms from the Congo and Zambezi Basins—Methodologies and identification of the genera. Abc Taxa 2016, 16, 1–353. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; English edition with updated taxonomy and added species; Cantonati, M., Kelly, M.G., Lange-Bertalot, H., Eds.; Koeltz Botanical Books: Schmitten, Germany, 2017; 942p. [Google Scholar]
- Poulíčkova, A.; Hašler, P. Aerophytic diatoms from caves in central Moravia (Czech Republic). Preslia 2007, 79, 185–204. [Google Scholar]
- Lowe, R.L.; Sherwood, A.R.; Ress, J.A. Freshwater species of Achnanthes Bory from Hawaii. Diatom Res. 2009, 24, 327–340. [Google Scholar] [CrossRef]
- Tofilovska, S.; Wetzel, C.E.; Ector, L.; Levkov, Z. Observation on Achnanthes Bory sensu stricto (Bacillariophyceae) from subaerial habitats in Macedonia and comparison with the type material of A. coarctata (Brebisson ex W. Smith) Grunow, A. coarctata var. sinaensis Hustedt and A. intermedia Kutzing. Fottea 2014, 14, 15–42. [Google Scholar] [CrossRef] [Green Version]
- Lobo, E.; Wetzel, C.; Heinrich, C.; Schuch, M.; Taques, F.; Ector, L. Occurrence of a poorly known small-sized Nitzschia species in headwaters streams from southern Brazil. Nova Hedwig. Beih. 2018, 146, 229–240. [Google Scholar] [CrossRef]
- Ehrenberg, C.G. Mikrogeologie. Das Erden und Felsen schaffende Wirken des Unsichtbar Kleinen Selbstständigen Lebens auf der Erde; Leopold Voss: Leipzig, Germany, 1854; 374p. [Google Scholar]
- Zimmermann, C. I Contribuição para o estudo das diatomaceas dos Estados Unidos do Brazil. Brotéria Série Botânica 1913, 11, 49–164. [Google Scholar]
- Zimmermann, C. II Contribuição para o estudo das diatomaceas dos Estados Unidos do Brazil. Brotéria Série Botânica 1915, 13, 37–59, 65–71. [Google Scholar]
- Zimmermann, C. IV Contribuição para o estudo das diatomaceas dos Estados Unidos do Brazil. Brotéria Série Botânica 1916, 14, 85–103. [Google Scholar]
- Zimmermann, C. V Contribuição para o estudo das diatomaceas dos Estados Unidos do Brazil. Brotéria Série Botânica 1916, 14, 130–157. [Google Scholar]
- Zimmermann, C. Algumas diatomaceas novas ou curiosas. Brotéria Série Botânica 1918, 16, 84–95. [Google Scholar]
- Gomes, D.F.; Caldas, O.; da Silva, E.M.; Gell, P.A.; Williams, D.M. Father Zimmermann (1871–1950): The first Brazilian diatomist. Diatom Res. 2012, 27, 177–188. [Google Scholar] [CrossRef]
- Zimmerman, C. Algumas diatomaceas novas ou curiosas. Brotéria Série Botânica 1917, 15, 5–7. [Google Scholar]
- Ferrari, F.; Ludwig, T.A.V. Coscinodiscophyceae, Fragilariophyceae e Bacillariophyceae (Achnanthales) dos rios Ivaí, São João e dos Patos, bacia hidrográfica do rio Ivaí, município de Prudentópolis, PR, Brasil. Acta Bot. Bras. 2007, 21, 421–441. [Google Scholar] [CrossRef] [Green Version]
- De Faria, D.M.; Tremarin, P.I.; Ludwig, T.A.V. Diatomáceas perifíticas da represa Itaqui, São José dos Pinhais, Paraná: Fragilariales, Eunotiales, Achnanthales e Gomphonema Ehrenberg. Biota Neotrop. 2010, 10, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Da Silva-Lehmkuhl, A.M.; Ludwig, T.A.V.; Tremarin, P.I.; Vercellino, I.S. Diatomáceas perifíticas em um sistema eutrófico brasileiro (Reservatório do Iraí, estado do Paraná). Acta Bot. Bras. 2010, 24, 997–1016. [Google Scholar]
- Moresco, C.; Tremarin, P.I.; Ludwig, T.A.V.; Rodrigues, L. Diatomáceas perifíticas abundantes em três córregos com diferentes ações antrópicas em Maringá, PR, Brasil. Braz. J. Bot. 2011, 34, 359–373. [Google Scholar] [CrossRef] [Green Version]
- Bes, D.; Ector, L.; Torgan, L.C.; Lobo, E.A. Composition of the epilithic diatom flora from a subtropical river, Southern Brazil. Iheringia. Série Botânica 2012, 67, 93–125. [Google Scholar]
- Cavalcante, K.P.; Tremarin, P.I.; De Castro, E.C.; Tibiriçá, C.E.J.D.A.; Wojciechowski, J.; Ludwig, T.A.V. Epiphytic Eunotia (Bacillariophyceae) on Podostemum from Santa Catarina, southern Brazil, including new observations on morphology and taxonomy of some rare recorded species. Biota Neotrop. 2014, 14, e20140034. [Google Scholar] [CrossRef]
- Tremarin, P.I.; Straube, A.; Ludwig, T.A.V. Nupela (Bacillariophyceae) in littoral rivers from south Brazil, and description of six new species of the genus. Fottea 2015, 15, 77–93. [Google Scholar] [CrossRef] [Green Version]
- Faustino, S.B.; Fontana, L.; Bartozek, E.C.R.; Bicudo, C.E.D.M.; Bicudo, D.D.C. Composition and distribution of diatom assemblages from core and surface sediments of a water supply reservoir in Southeastern Brazil. Biota Neotrop. 2016, 16, e20150129. [Google Scholar] [CrossRef] [Green Version]
- Marra, R.C.; Tremarin, P.I.; Algarte, V.M.; Ludwig, T.V. Epiphytic diatoms (Diatomeae) from Piraquara II urban reservoir, Paraná state. Biota Neotrop. 2016, 16, e20160200. [Google Scholar] [CrossRef] [Green Version]
- Straube, A.; Tremarin, P.I.; Ludwig, T.A.V. Species of Luticola D.G. Mann (Bacillariophyceae) in the Atlantic Forest rivers from southern Brazil. Diatom Res. 2017, 32, 417–437. [Google Scholar] [CrossRef]
- Medeiros, G.; Amaral, M.W.W.; Ferreira, P.C.; Ludwig, T.V.; Bueno, N.C. Gomphonema Ehrenberg (Bacillariophyceae, Gomphonemataceae) of the São Francisco Falso River, Paraná, Brazil. Biota Neotrop. 2018, 18, e20170495. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, M.; Büdel, B.; Lange-Bertalot, H. Diatoms living inside the thallus of the green algal lichen Coenogonium linkii in Neotropical lowland rain forests. J. Phycol. 2004, 40, 70–73. [Google Scholar] [CrossRef]
- Marques Filho, E.P.; Karam, H.A.; Miranda, A.G.; França, J.R.A. Rio de Janeiro’s tropical urban climate. Urban Clim. News 2009, 32, 5–9. [Google Scholar]
- Pougy, N.; Martins, E.; Verdi, M.; De Oliveira, J.A.; Maurenza, D.; Amaro, R.; Martinelli, G. Urban forests and the conservation of threatened plant species: The case of the Tijuca National Park, Brazil. Nat. Conserv. 2014, 12, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Grunow, A. Algae. In Reise der österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859 unter den Befehlen des Commodore B. von Wüllerstorf-Urbair. Botanischer Theil. Erster Band. Sporenpflanzen; Fenzl, E., Ed.; Aus der Kaiserlich Königlichen Hof- und Staatsdruckerei in Commission bei Karl Gerold’s Sohn: Vienna, Austria, 1867; pp. 1–104. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H. Tropische Diatomeen in Südamerika, I. 700 überwiegend wenig bekannte oder neue Taxa repräsentativ als Elemente der neotropischen Flora [Tropical diatoms of South America, I. About 700 predominantly rarely known or new taxa representative of the neotropical flora]. Iconogr. Diatomol. 1998, 5, 1–695. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H. Tropical diatoms of South America II. Special remarks on biogeographic disjunction. Iconogr. Diatomol. 2007, 18, 1–876. [Google Scholar]
- Rumrich, U.; Lange-Bertalot, H.; Rumrich, M. Diatomeen der Anden. Von Venezuela bis Patagonien/Feuerland [Diatoms of the Andes. From Venezuela to Patagonia/Tierra del Fuego]. Iconogr. Diatomol. 2000, 9, 1–649. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis Teil. In Süβwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Band 2/Gustav Fischer: Stuttgart, Germany; Jena, Germany, 1991; 437p. [Google Scholar]
- Metzeltin, D.; Lange-Bertalot, H.; García-Rodríguez, F. Diatoms of Uruguay. Compared with other taxa from South America and elsewhere. Iconogr. Diatomol. 2005, 15, 1–736. [Google Scholar]
- Gandhi, H.P. Some New Diatoms from the Jog-Falls (Mysore State). J. R. Microsc. Soc. 1959, 79, 81–87. [Google Scholar] [CrossRef]
- Gandhi, H.P. The fresh-water diatomflora of Jog-Falls, Mysore State. Nova Hedwig. 1966, 11, 89–197. [Google Scholar]
- Lowe, R.L.; Kociolek, P.; Johansen, J.R.; Van de Vijver, B.; Lange-Bertalot, H.; Kopalová, K. Humidophila gen. nov., a new genus for a group of diatoms (Bacillariophyta) formerly within the genus Diadesmis: Species from Hawai’i, including one new species. Diatom Res. 2014, 29, 351–360. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Nakamura, Y.; Nakano, S.; Miyake, Y. Diatoms from Ishite Stream, near the Komenono Forest Research Center of Ehime University, Japan. Diatom 2007, 23, 29–48. [Google Scholar]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Russian Academy of Sciences: Yaroslavl, Russia, 2016; 804p. [Google Scholar]
- Morales, E.A.; Vis, M.L. Epilithic diatoms (Bacillariophyceae) from cloud forest and alpine streams in Bolivia, South America. Proc. Acad. Nat. Sci. Phila. 2007, 156, 123–155. [Google Scholar] [CrossRef]
- Morales, E.A.; Fernández, E.; Kociolek, P.J. Epilithic diatoms (Bacillariophyta) from cloud forest and alpine streams in Bolivia, South America 3: Diatoms from Sehuencas, Carrasco National Park, Department of Cochabamba. Acta Bot. Croat. 2009, 68, 263–283. [Google Scholar]
- Moser, G.; Lange-Bertalot, H.; Metzeltin, D. Insel der Endemiten. Geobotanisches Phänomen Neukaledonien [Island of endemics. New Caledonia—A geobotanical phenomenon]. Bibl. Diatomol. 1998, 38, 1–464. [Google Scholar]
- Kützing, F.T. Die Kieselschaligen Bacillarien Oder Diatomeen; Zu Finden bei W. Köhne: Nordhausen, Germany, 1844; 144p. [Google Scholar] [CrossRef] [Green Version]
- Johansen, J.R.; Lowe, R.; Gomez, S.R.; Kociolek, J.P.M.; Makosky, S.A. New algal species records for the Great Smoky Mountains National Park, U.S.A., with an annotated checklist of all reported algal species for the park. Arch. Hydrobiol. 2004, 111, 17–44. [Google Scholar] [CrossRef]
- Zidarova, R.; Kopalová, K.; Van der Vijver, B. Diatoms from the Antarctic Region: Maritime Antarctica. Iconogr. Diatomol. 2016, 24, 1–504. [Google Scholar]
- Foged, N. Freshwater diatoms in Sri Lanka (Ceylon). Bibl. Phycol. 1976, 23, 1–113. [Google Scholar]






| Sample Signature | 2015/1 | 2015/2 | 2015/3 |
|---|---|---|---|
| Sampling date | 29 March 2015 | 29 March 2015 | 3 April 2015 |
| Geographical coordinates | 22°57′09″ S 43°13′40″ W | 22°57′09″ S 43°13′40″ W | 22°57′39″ S 43°16′21″ W |
| Altitude (m a.s.l.) | 60 | 60 | 340 |
| Habitat | Mosses collected from concrete wall in Rio de Janeiro city | Lichens and mosses collected from Roystonea regia L. trunk, 1.5 m above ground level | Mosses collected from palm trunk growing in rainforest |
| Taxa | Length (µm) | Width (µm) | Striae Density (in 10 µm) | Areolae Density (in 10 µm) | References |
|---|---|---|---|---|---|
| Taxa from Genus Achnanthes Identified in Studied Samples | |||||
| Achnanthes pseudoinflata sp. nov. | 18.1–38.5 | 6.9–9.6 | 15–17 | 18–22 | This study [Supplementary Materials Table S1] |
| Achnanthes coarctata | 31.1–36.4 | 8.5–9 | 11 | 14–15 | This study [Table S2] |
| 17–48 | 6–15 | 10–14 | 14–18 | [34] | |
| Achnanthes mauiensis | 23.2–32.4 | 6.3–6.4 | 15–16 | ~21 | This study [Table S3] |
| 20–28 | 5–7.5 | 15–19 | 20 | [8] | |
| Achnanthes inflata var. gibba | 24.3–49.5 | 10–12.7 | 12–13 | 18–19 R-valve 16–18 p-valve | This study [Table S4] |
| 34–40 | 14.3–15 | 10–12 | 18–19 | [39] | |
| Achnanthes inflata | 31–62.4 | 11.5–17.7 | 9–12 R-valve 9–13 p-valve | 12 R-valve 10–11 p-valve | Grunow’s original material [Table S5] (New Zealand) |
| 30–96 | 10–18 | 8–13 | 9–12 | [34] | |
| 30–65 | 10–20 | 10–13 | 11–12 | [38] | |
| 61.5–69 | 13.5–16.3 | 9–11 | No data | [8] | |
| 39–60 | 14–15 | 9–10 | 10–14 | [42] | |
| 30–96 | 10–21.1 | 9–12 | 9–12 | [43] | |
| Other Taxa Similar to Achnanthes pseudoinflata sp. nov. | |||||
| Achnanthes elata | 22–86 | 11–19 | 8–9 R-valve 9–10 p-valve | 10–11 | [39] |
| No data | 14–17 | ~10 R-valve 8–9 p-valve | 8–9 | [38] | |
| Achnanthes elata var. curvula | 60–68 | 19–21 | 8–9 R-valve 9–10 p-valve | No data | [39] |
| 88.9 | 17.8 | 8.5 R-valve 8 p-Valve | 8–9 | [40] | |
| Achnanthes inflata var. javanica | 70–81 | 22–22.5 | 8–9 R-valve 9–9.5 p-valve | 9–10 R-valve 10 p-valve | [40] |
| Achnanthes inflatagrandis | 82–100 | 27–30 | 7–8 | 8 | [38] |
| Achnanthes longboardia | 19–50.5 | 8–10.5 | 13–15 R-valve 12–14 p-valve | 18–19 | [8] |
| Achnanthes subelata | 30–43 | 9.5–12 | 11.5–13 R-valve 10–11 p-valve | ~12 | [38] |
| Achnanthes tumescens | 22–36.5 | 6.5–8.5 | 15–17 | 16 | [8] |
| Achnanthes cf. inflata | 30–40 | 12–13 | No data | No data | [36] |
| Achnanthes sp. cf. inflatagrandis | 56–64 | 16–20 | No data | No data | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak, M.; Peszek, Ł.; Skoczylas, Ł.; Noga, T.; Ector, L.; Wetzel, C.E. Achnanthes Bory Sensu Stricto (Bacillariophyta) from Terrestrial Habitats of Rio de Janeiro (Brazil), with Description of Achnanthes pseudoinflata sp. nov. Diversity 2020, 12, 375. https://doi.org/10.3390/d12100375
Rybak M, Peszek Ł, Skoczylas Ł, Noga T, Ector L, Wetzel CE. Achnanthes Bory Sensu Stricto (Bacillariophyta) from Terrestrial Habitats of Rio de Janeiro (Brazil), with Description of Achnanthes pseudoinflata sp. nov. Diversity. 2020; 12(10):375. https://doi.org/10.3390/d12100375
Chicago/Turabian StyleRybak, Mateusz, Łukasz Peszek, Łukasz Skoczylas, Teresa Noga, Luc Ector, and Carlos E. Wetzel. 2020. "Achnanthes Bory Sensu Stricto (Bacillariophyta) from Terrestrial Habitats of Rio de Janeiro (Brazil), with Description of Achnanthes pseudoinflata sp. nov." Diversity 12, no. 10: 375. https://doi.org/10.3390/d12100375
APA StyleRybak, M., Peszek, Ł., Skoczylas, Ł., Noga, T., Ector, L., & Wetzel, C. E. (2020). Achnanthes Bory Sensu Stricto (Bacillariophyta) from Terrestrial Habitats of Rio de Janeiro (Brazil), with Description of Achnanthes pseudoinflata sp. nov. Diversity, 12(10), 375. https://doi.org/10.3390/d12100375