Grassland Management Affects Vegetation Structure, Bats and Their Beetle Prey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farming Data
2.2. Carabid Beetles
2.3. Dung Beetles
2.4. Bat Activity
2.5. Vegetation Sampling
2.6. Data Processing
2.7. Statistical Analyses
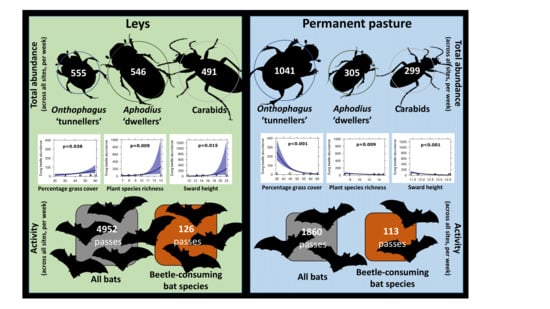
3. Results
3.1. Vegetation Structure
3.2. Beetles
3.3. Bats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat. Utilised Agricultural Area by Categories. 18 August 2018. Available online: https://ec.europa.eu/eurostat/tgm/refreshTableAction.do?tab=table&plugin=1&pcode=tag00025 (accessed on 18 September 2020).
- Defra. Farming Statistics: Final Crop Aras, Yields, Livestock Populations and Agricultural Workforce at June 2019-United Kingdom; Defra: London, UK, 2019.
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef] [Green Version]
- Flohre, A.; Fischer, C.; Aavik, T.; Bengtsson, J.; Berendse, F.; Bommarco, R.; Ceryngier, P.; Clement, L.W.; Dennis, C.; Eggers, S. Agricultural intensification and biodiversity partitioning in European landscapes comparing plants, carabids, and birds. Ecol. Appl. 2011, 21, 1772–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, R.; Norton, L.; Feber, R.; Johnson, P.; Chamberlain, D.E.; Joys, A.C.; Mathews, F.; Stuart, R.; Townsend, M.; Manley, W. Benefits of organic farming to biodiversity vary among taxa. Biol. Lett. 2005, 1, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic. Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Hutton, S.A.; Giller, P.S. The effects of the intensification of agriculture on northern temperate dung beetle communities. J. Appl. Ecol. 2003, 40, 994–1007. [Google Scholar] [CrossRef]
- Liu, M.; Ussiri, D.A.N.; Lal, R. Soil Organic Carbon and Nitrogen Fractions under Different Land Uses and Tillage Practices. Commun. Soil Sci. Plant Anal. 2016, 47, 1528–1541. [Google Scholar] [CrossRef]
- Powlson, D.S.; Bhogal, A.; Chambers, B.J.; Coleman, K.; Macdonald, A.J.; Goulding, K.W.T.; Whitmore, A.P. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Zhan, L.; Li, S.; Xu, Y.; Zhang, X.; Pei, X.; Pan, F.; Zhang, S.; Chen, P. Soil fauna community in the black soil of Northeast China under different tillage systems. Acta Agric. Scand. Sec. B Soil Plant Sci. 2014, 64, 462–469. [Google Scholar] [CrossRef]
- Henderson, I.G.; Ravenscroft, N.; Smith, G.; Holloway, S. Effects of crop diversification and low pesticide inputs on bird populations on arable land. Agric. Ecosyst. Environ. 2009, 129, 149–156. [Google Scholar] [CrossRef]
- Robinson, R.A.; Wilson, J.D.; Crick, H.Q.P. The importance of arable habitat for farmland birds in grassland landscapes. J. Appl. Ecol. 2001, 38, 1059–1069. [Google Scholar] [CrossRef]
- Seidl, M.; González, E.; Kadlec, T.; Saska, P.; Knapp, M. Temporary non-crop habitats within arable fields: The effects of field defects on carabid beetle assemblages. Agric. Ecosyst. Environ. 2020, 293, 106856. [Google Scholar] [CrossRef]
- Emmerson, M.; Morales, M.B.; Oñate, J.J.; Batáry, P.; Berendse, F.; Liira, J.; Aavik, T.; Guerrero, I.; Bommarco, R.; Eggers, S.; et al. Chapter Two—How Agricultural Intensification Affects Biodiversity and Ecosystem Services. In Advances in Ecological Research; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 55, pp. 43–97. [Google Scholar]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Threlfall, C.G. Urbanisation and Its Effects on Bats—A Global Meta-Analysis. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C., Kingston, T., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–33. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Vaughan, N. Bat activity and species richness on organic and conventional farms: Impact of agricultural intensification. J. Appl. Ecol. 2003, 40, 984–993. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species, Version 2020-2; 2020. Available online: https://www.iucnredlist.org (accessed on 12 July 2020).
- Mathews, F.; Kubasiewicz, L.M.; Gurnell, J.; Harrower, C.A.; McDonald, R.A.; Shore, R.F. A Review of the Population and Conservation Status of British Mammals. A report by the Mammal Society under contract to Natural England, Natural Resources Wales and Scottish Natural Heritage; Natural England: Peterborough, UK, 2018.
- Vaughan, N. The diets of British bats (Chiroptera). Mamm. Rev. 1997, 27, 77–94. [Google Scholar] [CrossRef]
- Asteraki, E. The potential of carabid beetles to control slugs in grass/clover swards. Entomophaga 1993, 38, 193–198. [Google Scholar] [CrossRef]
- Oberholzer, F.; Frank, T. Predation by the Carabid Beetles Pterostichus melanarius and Poecilus cupreus on Slugs and Slug Eggs. Biocontrol Sci. Technol. 2003, 13, 99–110. [Google Scholar] [CrossRef]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Jarosik, V. Ground beetles (Carabidae) as seed predators. EJE 2013, 100, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Scholtz, C.H.; Janeau, J.-L.; Grellier, S.; Podwojewski, P. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Appl. Soil. Ecol. 2010, 46, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Manning, P.; Slad, E.M.; Benyon, S.A.; Lewis, O.T. Effect of dung beetle species richness and chemical perturbation on multiple ecosystem functions. Ecol. Entomol. 2017, 42, 577–586. [Google Scholar] [CrossRef]
- Grønvold, J.; Sommer, C.; Hotter, P.; Nansen, P. Reduced Splash Dispersal of Bovine Parasitic Nematodes from Cow Pats by the Dung Beetle Diastellopalpus quinquedens. J. Parasitol. 1992, 78, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.S.; Lee, J.-H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil. Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Sands, B.; Wall, R. Sustained parasiticide use in cattle farming affects dung beetle functional assemblages. Agric. Ecosyst. Environ. 2018, 265, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Maskell, L.C.; Norton, L.R.; Smart, S.M.; Scott, R.; Carey, P.D.; Murphy, J.; Chamberlain, P.M.; Wood, C.M.; Bunce, R.G.H.; Barr, C.J. Vegetation Plots Handbook; Centre for Ecology and Hydrology: Bailrigg, UK, 2008. [Google Scholar]
- Forsythe, T. Common Ground Beetles Naturalist’s Handbook 8; Richmond Publishing: Oxford, UK, 1987; Volume 74, pp. 223–236. [Google Scholar]
- Jessop, L. Dung Beetles and Chafers; Royal Entomological Society of London: London, UK, 1986; Volume 5. [Google Scholar]
- Luff, M.L. The Carabidae (Ground Beetles) of Britain and Ireland; Field Studies Council: Shrewsbury, UK, 2007. [Google Scholar]
- Russ, J. British Bat Calls: A Guide to Species Identification; Pelagic Publishing: Exeter, UK, 2012. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; 3.5.3; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Doube, B.M. A functional classification for analysis of the structure of dung beetle assemblages. Ecol. Entomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- Adl, S.M.; Coleman, D.C.; Read, F. Slow recovery of soil biodiversity in sandy loam soils of Georgia after 25 years of no-tillage management. Agric. Ecosyst. Environ. 2006, 114, 323–334. [Google Scholar] [CrossRef]
- Anken, T.; Weisskopf, P.; Zihlmann, U.; Forrer, H.; Jansa, J.; Perhacova, K. Long-term tillage system effects under moist cool conditions in Switzerland. Soil Tillage Res. 2004, 78, 171–183. [Google Scholar] [CrossRef]
- Hatten, T.D.; Bosque-Pérez, N.A.; Labonte, J.R.; Guy, S.O.; Eigenbrode, S.D. Effects of Tillage on the Activity Density and Biological Diversity of Carabid Beetles in Spring and Winter Crops. Environ. Entomol. 2007, 36, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Peigné, J.; Cannavacuolo, M.; Gautronneau, Y.; Aveline, A.; Giteau, J.L.; Cluzeau, D. Earthworm populations under different tillage systems in organic farming. Soil Tillage Res. 2009, 104, 207–214. [Google Scholar] [CrossRef]
- Holland, J.; Luff, M. The effects of agricultural practices on Carabidae in temperate agroecosystems. Integr. Pest Manag. Rev. 2000, 5, 109–129. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Norris, S.L.; Murray, P.J. Impact of Grassland Reseeding, Herbicide Spraying and Ploughing on Diversity and Abundance of Soil Arthropods. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menalled, F.D.; Smith, R.G.; Dauer, J.T.; Fox, T.B. Impact of agricultural management on carabid communities and weed seed predation. Agric. Ecosyst. Environ. 2007, 118, 49–54. [Google Scholar] [CrossRef]
- Clark, S.; Szlavecz, K.; Cavigelli, M.A.; Purrington, F. Ground Beetle (Coleoptera: Carabidae) Assemblages in Organic, No-Till, and Chisel-Till Cropping Systems in Maryland. Environ. Entomol. 2006, 35, 1304–1312. [Google Scholar] [CrossRef]
- Jabbour, R.; Pisani-Gareau, T.; Smith, R.G.; Mullen, C.; Barbercheck, M. Cover crop and tillage intensities alter ground-dwelling arthropod communities during the transition to organic production. Renew. Agric. Food Syst. 2016, 31, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Jerrentrup, J.S.; Wrage-Mönnig, N.; Röver, K.-U.; Isselstein, J. Grazing intensity affects insect diversity via sward structure and heterogeneity in a long-term experiment. J. Appl. Ecol. 2014, 51, 968–977. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Contrasting responses of plant and insect diversity to variation in grazing intensity. Biol. Conserv. 2002, 106, 293–302. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Grazing Intensity and the Diversity of Grasshoppers, Butterflies, and Trap-Nesting Bees and Wasps. Conserv. Biol. 2002, 16, 1570–1580. [Google Scholar] [CrossRef]
- Brose, U. Bottom-up control of carabid beetle communities in early successional wetlands: Mediated by vegetation structure or plant diversity? Oecologia 2003, 135, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.J. Pest Lepidoptera of Europe: With Special Reference to the British Isles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1984; Volume 31. [Google Scholar]
- Ransome, R.D. The Management of Feeding Areas for Greater Horseshoe Bats; English Nature: Peterborough, UK, 1996.
- Finch, D.; Schofield, H.; Mathews, F. Habitat Associations of Bats in an Agricultural Landscape: Linear Features Versus Open Habitats. Animals 2020, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Lacoeuilhe, A.; Machon, N.; Julien, J.-F.; Kerbiriou, C. The relative effects of local and landscape characteristics of hedgerows on bats. Diversity 2018, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.L.; Harris, S. Foraging habitat preferences of vespertilionid bats in Britain. J. Appl. Ecol. 1996, 33, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Jones, G. Prey Selection by the Greater Horseshoe Bat (Rhinolophus ferrumequinum): Optimal Foraging by Echolocation? J. Anim. Ecol. 1990, 59, 587–602. [Google Scholar] [CrossRef]
- Beck, A. Fecal analyses of European bat species. Myotis 1995, 32, 109–119. [Google Scholar]
- Robinson, M.F.; Stebbings, R.E. Food of the serotine bat, Eptesicus serotinus—Is faecal analysis a valid qualitative and quantitative technique? J. Zool. 1993, 231, 239–248. [Google Scholar] [CrossRef]
- Zukal, J.; Gajdošik, M. Diet of Eptesicus serotinus in an agricultural landscape. Vespertilio 2012, 16, 357–363. [Google Scholar]
- Kervyn, T.; Libois, R. The Diet of the serotine bat A Comparison between rural and urban environments. Belg. J. Zool. 2008, 138, 41–49. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M.; Norton, L.; Mathews, F. Grassland Management Affects Vegetation Structure, Bats and Their Beetle Prey. Diversity 2020, 12, 406. https://doi.org/10.3390/d12100406
Anderson M, Norton L, Mathews F. Grassland Management Affects Vegetation Structure, Bats and Their Beetle Prey. Diversity. 2020; 12(10):406. https://doi.org/10.3390/d12100406
Chicago/Turabian StyleAnderson, Max, Lisa Norton, and Fiona Mathews. 2020. "Grassland Management Affects Vegetation Structure, Bats and Their Beetle Prey" Diversity 12, no. 10: 406. https://doi.org/10.3390/d12100406
APA StyleAnderson, M., Norton, L., & Mathews, F. (2020). Grassland Management Affects Vegetation Structure, Bats and Their Beetle Prey. Diversity, 12(10), 406. https://doi.org/10.3390/d12100406