Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Algal Assemblages
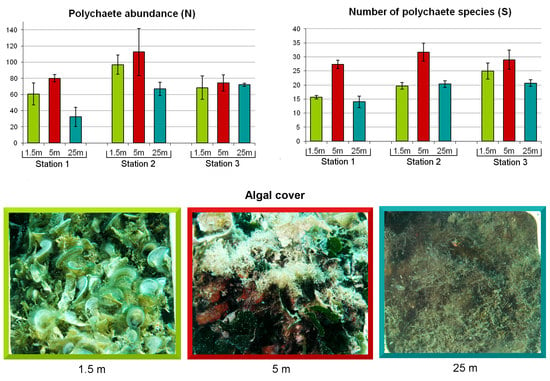
3.2. Composition and Diversity of Polychaete Assemblages
3.3. Patterns of Variation of Polychaete Assemblages Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buljan, M.; Zore-Armanda, M. Oceanographical properties of the Adriatic Sea. Oceanogr. Mar. Biol. Annu. Rev. 1976, 14, 11–98. [Google Scholar]
- McKinney, F.K. The Northern Adriatic Ecosystem: Deep Time in a Shallow Sea; Columbia University Press: New York, NY, USA, 2007; 328p. [Google Scholar]
- Degobbis, D.; Gilmartin, M. Nitrogen, phosphorus and biogenic silicon budgets for the northern Adriatic Sea. Oceanol. Acta 1990, 13, 31–45. [Google Scholar]
- Boero, F.; Bonsdorff, E. A conceptual framework for marine biodiversity and ecosystem functioning. Mar. Ecol Evol Perspect 2007, 28 (Suppl. 1), 134–145. [Google Scholar] [CrossRef]
- Mikac, B.; Semprucci, F.; Guidi, L.; Ponti, M.; Abbiati, M.; Balsamo, M.; Dovgal, I. Newly discovered associations between peritrich ciliates (Ciliophora: Peritrichia) and scale polychaetes (Annelida: Polynoidae and Sigalionidae) with a review of polychaete–peritrich epibiosis. Zool. J. Linn. Soc 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; Lasram, F.B.R.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under siege: Spatial overlap between marine biodiversity, cumulative threats and marine reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2012, 5. [Google Scholar] [CrossRef] [Green Version]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative Human Impacts on Mediterranean and Black Sea Marine Ecosystems: Assessing Current Pressures and Opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for Community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, L 164, 25.6.2008, 19–40.
- Knox, G.A. The role of Polychaetes in benthic soft-bottom communities. In Essays on Polychaetous Annelids in Memory of Olga Hartmann; Reish, D., Fauchald, K., Eds.; Allan Hancock Foundation: Los Angeles, CA, USA, 1977; pp. 547–604. [Google Scholar]
- Musco, L. Ecology and diversity of Mediterranean hard-bottom Syllidae (Annelida): A community-level approach. Mar. Ecol Prog. Ser. 2012, 461, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Giangrande, A.; Licciano, M.; Musco, L. Polychaetes as environmental indicators revisited. Mar. Pollut Bull. 2005, 50, 1153–1162. [Google Scholar] [CrossRef]
- Aguado-Giménez, F.; Gairin, J.I.; Martinez-Garcia, E.; Fernandez-Gonzalez, V.; Ballester Molto, M.; Cerezo-Valverde, J.; Sanchez-Jerez, P. Application of taxocene surrogation and taxonomic sufficiency concepts to fish farming environmental monitoring. Comparison of BOPA index versus polychaete assemblage structure. Mar. Environ. Res. 2015, 103, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musco, L.; Terlizzi, A.; Licciano, M.; Giangrande, A. Taxonomic structure and the effectiveness of surrogates in environmental monitoring: A lesson from polychaetes. Mar. Ecol Prog. Ser. 2009, 383, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Soares-Gomes, A.; Mendes, C.L.T.; Tavares, M.; Santi, L. Taxonomic sufficiency of polychaete taxocenes for estuary monitoring. Ecol Indic. 2012, 15, 149–156. [Google Scholar] [CrossRef]
- Aleffi, F.; Bettoso, N.; Solis-Weiss, V. Spatial distribution of soft–bottom polychaetes along western coast of the northern Adriatic Sea (Italy). Ann. Ser. Hist Nat. 2003, 13, 211–222. [Google Scholar]
- Amoureux, L. Inventaire d’une petit collection d’Annélides Polychètes des parages sud de Rovinj (Haute–Adriatique). Thalass. Jugosl. 1976, 12, 381–390. [Google Scholar]
- Amoureux, L. Annélides Polychètes recueillies par D. Zavodnik. Thalass. Jugosl. 1983, 19, 1–6. [Google Scholar]
- Castelli, A.; Prevedelli, D. Effetto del fenomeno delle mucillaggini dell’estate 1989 sul popolamento a policheti di un microhabitat salmastro preso Punta Marina (Ravenna). Biol. Mar. Meditsuppl. Al Not. S.I.B.M. 1993, 1, 35–38. [Google Scholar]
- Crema, R.; Prevedelli, D.; Valentini, A.; Castelli, A. Recovery of the macrozoobenthic community of the Comacchio lagoon system (Northern Adriatic Sea). Ophelia 2000, 52, 143–152. [Google Scholar] [CrossRef]
- Fauvel, P. Annélides Polychètes de Rovigno d’Istria. Thalassia 1934, 1, 1–78. [Google Scholar]
- Fauvel, P. Annelida Polychaeta della Laguna di Venezia. Bollettino. R. Com. Talassogr. Ital. 1938, 246, 1–27. [Google Scholar]
- Fauvel, P. Annélides Polychètes de la Haute Adriatique. Thalassia 1940, 4, 1–24. [Google Scholar]
- Gamulin-Brida, H.; Požar, A.; Zavodnik, D. Contributions aux recherches sur la bionomie benthique des fonds meubles de l’Adriatique du nord (II). Biol. Glas 1968, 21, 157–201. [Google Scholar]
- Gillet, P. Annélides Polychètes des fonds meubles du Canal de Lim prés de Rovinj (Yugoslavie). Thalassia Jugosl. 1986, 22/21, 127–138. [Google Scholar]
- Maggiore, F.; Keppel, E. Biodiversity and distribution of polychaetes and molluscs along the Dese estuary (Lagoon of Venice, Italy). Hydrobiologia 2007, 588, 189–203. [Google Scholar] [CrossRef]
- Mikac, B.; Musco, L.; Đakovac, T.; Giangrande, A.; Terlizzi, T. Long–term changes in North Adriatic soft–bottom polychaete assemblages following a dystrophic crisis. Ital. J. Zool. 2011, 78, 304–316. [Google Scholar] [CrossRef]
- Mistri, M.; Fano, A.E.; Ghion, F.; Rossi, R. Disturbance and community pattern of polychaetes inhabiting Valle Magnavacca (Valli di Comacchio, Northern Adriatic Sea, Italy). Mar. Ecol. Pszni 2002, 23, 31–49. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Savini, D.; Forni, G. Macrobenthos community structural changes off Cesenatico coast (Emilia Romagna, Northern Adriatic), a six–year monitoring programme. Sci. Tot Environ. 2005, 353, 317–328. [Google Scholar] [CrossRef]
- Prevedelli, D.; Bellucci, L.G.; Simononi, R.; Ansaloni, I.; Frignani, M.; Ravaioli, M.; Castelli, A. Macrobenthos and environmental characteristics of the Venice lagoon. Atti. Soc. Nat. Mat. Modena 2007, 138, 151–161. [Google Scholar]
- Zahtila, E. Offshore polychaete fauna in the northern Adriatic with trophic characteristic. Period. Biol. 1997, 99, 213–217. [Google Scholar]
- Zavodnik, D.; Vidaković, J.; Amoureux, L. Contribution to sediment macrofauna in the area of Rovinj (North Adriatic Sea). Cah Biol. Mar. 1985, 26, 431–444. [Google Scholar]
- Amoureux, L. Annélides Polychètes de l’îlot Banjole (prés de Rovinj, haute–Adriatique). Cah Biol. Mar. 1975, 16, 231–244. [Google Scholar]
- Amoureux, L.; Katzmann, W. Note faunistique et écologique sur une collection d’Annélides Polychètes de substrats rocheux circalittoraux de la région de Rovinj (Yougoslavie). Zool Anz 1971, 186, 114–122. [Google Scholar]
- Casellato, S.; Masiero, L.; Sichirollo, E.; Soresi, S. Hidden secrets of the Northern Adriatic: ‘‘Tegnue’’, peculiar reefs. Centr. Eur. J. Biol. 2007, 2, 122–136. [Google Scholar] [CrossRef]
- Casellato, S.; Stefanon, A. Coralligenous habitat in the northern Adriatic Sea: An overview. Mar. Ecol. 2008, 29, 321–341. [Google Scholar] [CrossRef]
- Katzmann, W. Polychaeten (Errantier, Sedentarier) aus nord—Adriatischen Cystoseira—Beständen und deren Epiphyten. Oecologia 1971, 8, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, V.; Mavrič, B.; Orlando-Bonaca, M.; Lipej, L. Rocky macrozoobenthos mediolittoral community in the Gulf of Trieste (North Adriatic) along a gradient of hydromorphological modifications. Acta Adriat. 2013, 54, 67–86. [Google Scholar]
- Pitacco, V.; Orlando-Bonaca, M.; Mavrič, B.; Lipej, L. Macrofauna associated with a bank of Cladocora caespitosa (Anthozoa, scleractinia) in the Gulf of Trieste (northern Adriatic). Ann. Ser. Hist Nat. 2014, 24, 1–14. [Google Scholar]
- Zavodnik, D. Prispevk k poznavanju naselja Cystoseira barbata (Good. & Wood.) C. Ag. v severnem Jadranu. Biol. Vest 1965, 13, 87–101. [Google Scholar]
- Zavodnik, D. Dinamika litoralnega fitala na zahodnoistrski obali. Razpr. Slov. Akad. Znan. Umet. 1967, 10, 5–71. [Google Scholar]
- Zavodnik, D.; Legac, M.; Gluhak, T. An account of the marine fauna of Pag Island (Adriatic Sea, Croatia). Nat. Croat. 2006, 15, 65–107. [Google Scholar]
- Mikac, B.; Musco, L. Faunal and biogeographic analysis of Syllidae (Polychaeta) from Rovinj (Croatia, northern Adriatic Sea). Sci. Mar. 2010, 74, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Mikac, B.; Liciano, M.; Giangrande, A. Sabellidae and Fabriciidae (Polychaeta) of the Adriatic Sea with particular retrospect to the Northern Adriatic and the description of two new species. J. Mar. Biol. Assoc. Uk 2013, 93, 1511–1524. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Bulleri, F.; Cinelli, F. The interplay of physical and biological factors in maintaining midshore and low-shore assemblages on rocky coasts in the north-west Mediterranean. Oecologia 2000, 123, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Çinar, M.E. Ecological features of Syllidae (Polychaeta) from shallow-water benthic environments of the Aegean Sea, eastern Mediterranean. J. Mar. Biol. Ass Uk 2003, 83, 737–745. [Google Scholar] [CrossRef]
- Fraschetti, S.; Terlizzi, A.; Benedetti-Cecchi, L. Patterns of distribution of rocky marine assemblages: Evidence of relevant scales of variation. Mar. Ecol. Prog. Ser. 2005, 296, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Irving, A.D.; Connell, S.D. Sedimentation and light penetration interact to maintain heterogeneity of subtidal habitats: Algal versus invertebrate dominated assemblages. Mar. Ecol. Prog. Ser. 2002, 245, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, A.; Anderson, M.J.; Fraschetti, S.; Benedetti-Cecchi, L. Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths. Mar. Ecol. Prog. Ser. 2007, 332, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Underwood, A.J.; Chapman, M.G. Scales of spatial patterns of distribution of intertidal invertebrates. Oecologia 1996, 107, 212–224. [Google Scholar] [CrossRef]
- Dorgham, M.M.; Hamdy, R.; El-Rashidy, H.H.; Atta, M.M.; Musco, L. Distribution patterns of shallow water polychaetes (Annelida) along the coast of Alexandria, Egypt (eastern Mediterranean). Mediterr. Mar. Sci. 2014, 15, 635–649. [Google Scholar] [CrossRef] [Green Version]
- Giangrande, A. Polychaete zonation and its relation to algal distribution down a vertical cliff in the western Mediterranean (Italy): A structural analysis. J. Exp. Mar. Biol. Ecol. 1988, 120, 263–276. [Google Scholar] [CrossRef]
- Somaschini, A. Policheti della biocenosi ad alghe fotofile (Facies a Corallina elongata) nel Lazio settentrionale. Atti Sac. Toscana Sci. Nat. Mem Ser. B 1988, 95, 83–94. [Google Scholar]
- Iveša, L. Dinamika Popluacija Makrofitobentosa na Hridinastim Dnima uz Zapadnu Obalu Istre. Ph.D. Thesis, Faculty of Science, University of Zagreb, Zagreb, Croatia, 2005. [Google Scholar]
- Iveša, Lj.; Lyons, D.M.; Devescovi, M. Assessment of the ecological status of north-eastern Adriatic coastal waters (Istria, Croatia) using macroalgal assemblages for the European Union Water Framework Directive. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 14–23. [Google Scholar] [CrossRef]
- Boudouresque, C. Méthodes d’étude qualitative et quantitative du benthos (en particulier du phytobenthos). Tethys 1971, 3, 79–104. [Google Scholar]
- Casoli, E.; Bonifazi, A.; Ardizzone, G.; Gravina, M.F. How algae influence sessile marine organisms: The tubeworms case of study. Estuar. Coast. Shelf Sci. 2016, 178, 12–20. [Google Scholar] [CrossRef]
- Fraschetti, S.; Giangrande, A.; Terlizzi, A.; Miglietta, M.P.; Della Tommasa, L.; Boero, F. Spatio-temporal variation of hydroids and polychaetes associated with Cystoseira amentacea (Fucales: Phaeophyceae). Mar. Biol. 2002, 140, 949–957. [Google Scholar] [CrossRef]
- Cormaci, M.; Furnari, G.; Giaccone, G. Macrophytobenthos. In Mediterranean Marine Benthos: A Manual of Methods for Its Sampling and Study; Gambi, M.C., Dappiano, M., Eds.; Biol. Mar. Medit. 11 (1), Chapter 7; Società Italiana di Biologia Marina: Genova, Italy, 2004; pp. 217–246. [Google Scholar]
- Littler, M.M.; Littler, D.S. The evolution of thallus form and survival strateges in benthic marine macroalgae: Field and laboratory tests of a functional form model. Am. Nat. 1980, 116, 5–44. [Google Scholar] [CrossRef] [Green Version]
- Littler, M.M.; Littler, D.S. Relationships between macroalgal functional form groups and substrata stability in a subtropical rocky-intertidal system. J. Exp. Mar. Biol. Ecol. 1984, 74, 13–34. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 256p. [Google Scholar]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. In Biodiversity: Measurement and Estimation; Hawksworth, D.L., Ed.; The Royal Society: London, UK, 1994; pp. 101–118. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. J. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R. Nonparametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 17–143. [Google Scholar] [CrossRef]
- Anderson, M.J. DISTLM Forward: A FORTRAN Computer Program to Calculate a Distance-Based Multivariate Analysis for a Linear Model Using Forward Selection; Department of Statistics, University of Auckland: Auckland, New Zealand, 2003. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; 192p. [Google Scholar]
- Pérès, J.M.; Gamulin-Brida, H. Biološka Oceanografija. Bentos. Bentoska Bionomija Jadranskog Mora; Školska knjiga: Zagreb, Croatia, 1973; 493p. [Google Scholar]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N.V. An insight to ecological evaluation index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- Munda, I.M. Changes in the benthic algal associations of the vicinity of Rovinj (Istrian coast, North Adriatic) caused by organic wastes. Acta Adriat 1980, 21, 299–332. [Google Scholar]
- Mikac, B. A sea of worms: Polychaete checklist of the Adriatic Sea. Zootaxa 2015, 3943, 1–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Çinar, M.E.; García Raso, J.E.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Medit Mar. Sci. 2010, 11/2, 381–493. [Google Scholar] [CrossRef]
- Amoureux, L. Annélides Polychètes de l’Adriatique. Thalass. Jugosl. 1983, 19, 15–21. [Google Scholar]
- Zavodnik, D.; Kovačić, M. Index of marine fauna in Rijeka Bay (Adriatic Sea, Croatia). Nat. Croat. 2000, 9, 297–379. [Google Scholar]
- Ben-Eliahu, M.N. Polychaeta errantia of the Suez Canal. Isr. J. Zool. 1972, 21, 189–237. [Google Scholar]
- Çinar, M.E. Alien polychaete species (Annelida: Polychaeta) on the southern coast of Turkey (Levantine Sea, eastern Mediterranean), with 13 new records for the Mediterranean Sea. J. Nat. Hist 2009, 43, 2283–2328. [Google Scholar] [CrossRef]
- Barnich, R.; Fiege, D. The Aphroditoidea (Annelida: Polychaeta) of the Mediterranean Sea. Abh. Der. Senckenbergischen Nat. Ges. Frankfut Am. Main 2003, 559, 1–170. [Google Scholar]
- Chatzigeorgiou, G.; Faulwetter, S. Polychaetes from Two Subtidal Rocky Shores of the North Coast of Crete, Collected for the NaGISA Project 2007–2008. Version 1.8, 2020, Sampling Event Dataset. Available online: https://www.gbif.org/occurrence/1705514079 (accessed on 13 April 2020). [CrossRef]
- Giangrande, A.; Delos, A.L.; Fraschetti, S.; Musco, L.; Licciano, M.; Terlizzi, A. Polychaete assemblages along a rocky shore on the South Adriatic coast (Mediterranean Sea): Patterns of spatial distribution. Mar. Biol. 2003, 143, 1109–1116. [Google Scholar] [CrossRef]
- Giangrande, A.; Delos, A.L.; Musco, L.; Licciano, M.; Pierri, C. Polychaete assemblages of rocky shore along the South Adriatic coast (Mediterranean Sea). Cah Biol. Mar. 2004, 45, 85–95. [Google Scholar]
- Pleijel, F. Phylogeny and classification of Hesionidae (Polychaeta). Zool Scr. 1998, 27, 89–163. [Google Scholar] [CrossRef]
- Çinar, M.E.; Gönlügür-Demirci, G. Polychaete assemblages on shallow-water benthic habitats along the Sinop Peninsula (Black Sea, Turkey). Cah Biol. Mar. 2005, 46, 253–263. [Google Scholar]
- Gambi, M.C.; Musco, L.; Giangrande, A.; Badalamenti, F.; Micheli, F.; Kroeker, K.J. Distribution and functional traits of polychaetes in a CO2 vent system: Winners and losers among closely related species. Mar. Ecol. Prog. Ser. 2016, 550, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.; Dahl, K. Temporal, spatial and substrate-dependent variations of Danish hard-bottom macrofauna. Helgol. Mar. Res. 2002, 56, 159–168. [Google Scholar] [CrossRef]
- Serrano, A.; Preciado, I. Environmental factors structuring polychaete communities in shallow rocky habitats: Role of physical stress versus habitat complexity. Helgol Mar. Res. 2007, 61, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, L. The effects of sedimentation on rocky coast assemblages. Oceanogr Mar. Biol. Ann. Rev. 2003, 41, 161–236. [Google Scholar]
- Sardá, R. Polychaete communities related to plant covering in the midlittoral and infralittoral zones of the Balearic Islands (Western Mediterranean). Pszni Mar. Ecol. 1991, 12, 341–360. [Google Scholar] [CrossRef]
- López, E.; Viéitez, J.M. Polychaete assemblages on non-encrusting infralittoral algae from the Chafarinas Islands (SW Mediterranean). Cah Biol. Mar. 1999, 40, 375–384. [Google Scholar]
- Sanfilippo, R.; Rosso, A.; Sciuto, F.; Serio, D.; Catra, M.; Alongi, G.; Negri, M.P.; Leonardi, R.; Viola, A. Serpulid polychaetes from Cystoseira communities in the Ionian Sea, Mediterranean. Vie Et Milieu 2017, 67, 217–226. [Google Scholar]
- Tena, J.; Capaccioni-Azzati, R.; Torres-Gravila, F.J.; Garcia-Carrascosa, A.M. Polychaetes associated with different facies of the photophilic algal community in the Chafarinas archipelago (SW Mediterranean). Bull. Mar. Sci. 2000, 67, 55–72. [Google Scholar]
- Cacabelos, E.; Olabarria, C.; Incera, M.; Troncoso, J.S. Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuar Coast. Shelf Sci. 2010, 89, 43–52. [Google Scholar] [CrossRef]
- Falace, A.; Alongi, G.; Cormaci, M.; Furnari, G.; Curiel, D.; Cecere, E.; Petrocelli, A. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chem. Ecol. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Marchini, A.; Ragazzola, F.; Vasapollo, C.; Castelli, A.; Cerrati, G.; Gazzola, F.; Jiang, C.; Langeneck, J.; Manauzzi, M.C.; Musco, L.; et al. Intertidal Mediterranean Coralline Algae Habitat Is Expecting a Shift Toward a Reduced Growth and a Simplified Associated Fauna Under Climate Change. Front. Mar. Sci. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]






| PERMANOVA | PERMDISP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | Up | P (perm) | df1 | df2 | F | P (perm) | |
| st | 2 | 5442.3 | 2721.1 | 4.7539 | 9953 | 0.0192 | 2 | 24 | 2.4351 | 0.1038 | |
| N | de | 2 | 4607.2 | 2303.6 | 4.3316 | 6066 | 0.0995 | - | - | ||
| stxde | 4 | 2127.3 | 531.81 | 0.92908 | 9956 | 0.4792 | - | - | |||
| Res | 18 | 10303 | 572.41 | ||||||||
| st | 2 | 178.74 | 89.37 | 6.4866 | 9949 | 0.0076 | 2 | 24 | 0.3413 | 0.8379 | |
| S | de | 2 | 627.63 | 313.81 | 18.976 | 3825 | 0.0254 | 2 | 24 | 0.2366 | 0.7997 |
| stxde | 4 | 66.148 | 16.537 | 1.2003 | 9950 | 0.3431 | - | - | |||
| Res | 18 | 248 | 13.778 | ||||||||
| st | 2 | 101.95 | 50.974 | 7.9594 | 9955 | 0.0028 | 2 | 24 | 1.0783 | 0.4925 | |
| N1 | de | 2 | 282.77 | 141.38 | 9.7949 | 6091 | 0.0459 | 2 | 24 | 3.2631 | 0.0885 |
| stxde | 4 | 57.737 | 14.434 | 2.2538 | 9950 | 0.0995 | - | - | |||
| Res | 18 | 115.28 | 6.4043 | ||||||||
| st | 2 | 0.0809 | 0.0405 | 4.293 | 9950 | 0.0304 | 2 | 24 | 1.1321 | 0.4103 | |
| N10 | de | 2 | 0.0065 | 0.0032 | 0.25075 | 6086 | 0.7786 | - | - | ||
| stxde | 4 | 0.0517 | 0.0129 | 1.3718 | 9937 | 0.2792 | - | - | |||
| Res | 18 | 0.1696 | 0.0094 | ||||||||
| st | 2 | 7282.2 | 3641.1 | 2.5077 | 9900 | 0.0001 | 2 | 24 | 4.2513 | 0.0549 | |
| Stru | de | 2 | 26808 | 13404 | 4.3784 | 6114 | 0.0176 | 2 | 24 | 2.4735 | 0.1668 |
| stxde | 4 | 12246 | 3061.4 | 2.1084 | 9843 | 0.0001 | 8 | 18 | 8.0471 | 0.0122 | |
| Res | 18 | 26136 | 1452 | ||||||||
| Group | Species | Abund | Sim% | Sim/ SD | Contrib% | Cum% | ||
| A | Sim% | |||||||
| 1.5 m | Amphiglena mediterranea | 9.67 | 8.14 | 1.63 | 23.68 | 23.68 | ||
| 34.39% | Platynereis dumerilii | 6.11 | 5.41 | 1.48 | 15.72 | 39.40 | ||
| Nereis usticensis | 11.00 | 2.45 | 0.33 | 7.13 | 46.54 | |||
| Syllis rosea | 7.00 | 2.45 | 1.11 | 7.12 | 53.66 | |||
| Sphaerosyllis pirifera | 4.67 | 2.43 | 0.71 | 7.06 | 60.72 | |||
| Nereis pulsatoria | 3.11 | 2.35 | 1.05 | 6.85 | 67.56 | |||
| Exogone dispar | 2.89 | 1.62 | 0.65 | 4.70 | 72.26 | |||
| 5 m | Amphiglena mediterranea | 17.56 | 10.46 | 1.43 | 23.14 | 23.14 | ||
| 45.22% | Sphaerosyllis pirifera | 5.67 | 4.06 | 1.46 | 8.97 | 32.11 | ||
| Nereididae juv. indet. | 4.00 | 3.04 | 2.11 | 6.71 | 38.82 | |||
| Nereis sp. 1 | 4.56 | 2.92 | 1.37 | 6.46 | 45.28 | |||
| Exogone dispar | 3.67 | 2.79 | 2.13 | 6.17 | 51.45 | |||
| Syllis variegata | 3.33 | 2.55 | 1.33 | 5.63 | 57.08 | |||
| Platynereis dumerilii | 3.56 | 2.50 | 1.17 | 5.52 | 62.60 | |||
| Syllis prolifera | 5.33 | 2.12 | 0.71 | 4.69 | 67.29 | |||
| Syllis corallicola | 3.11 | 2.08 | 2.29 | 4.59 | 71.88 | |||
| 25 m | Syllis armillaris | 13.22 | 13.05 | 1.55 | 33.04 | 33.04 | ||
| 39.51% | Syllis gracilis | 6.67 | 8.38 | 1.88 | 21.21 | 54.25 | ||
| Sphaerosyllis pirifera | 8.78 | 5.93 | 0.98 | 15.01 | 69.27 | |||
| Syllis gerlachi | 4.11 | 3.65 | 1.44 | 9.24 | 78.50 | |||
| Groups | Species | Av. Ab | Av.Ab | Diss% | Diss/SD | Contrib% | Cum% | |
| B | Diss% | Group 1.5 | Group 5 | |||||
| 1.5 and 5 | Amphiglena mediterranea | 9.67 | 17.56 | 7.34 | 1.35 | 10.69 | 10.69 | |
| 68.67% | Nereis usticensis | 11.00 | 0.11 | 5.99 | 0.71 | 8.72 | 19.41 | |
| Syllis rosea | 7.00 | 0.44 | 4.01 | 0.58 | 5.83 | 25.24 | ||
| Syllis prolifera | 5.44 | 5.33 | 3.89 | 1.16 | 5.66 | 30.91 | ||
| Sphaerosyllis pirifera | 4.67 | 5.67 | 3.38 | 1.13 | 4.92 | 35.83 | ||
| Platynereis dumerilii | 6.11 | 3.56 | 2.53 | 1.21 | 3.68 | 39.51 | ||
| Nereis sp. 1 | 1.11 | 4.56 | 2.39 | 1.22 | 3.48 | 42.99 | ||
| Nereididae juv. indet. | 2.00 | 4.00 | 2.16 | 1.47 | 3.15 | 46.14 | ||
| Syllis variegata | 0.33 | 3.33 | 2.01 | 1.44 | 2.92 | 49.07 | ||
| Exogone dispar | 2.89 | 3.67 | 1.95 | 1.29 | 2.84 | 51.90 | ||
| Syllis corallicola | 1.00 | 3.11 | 1.89 | 1.69 | 2.75 | 54.65 | ||
| Syllis gerlachi | 0.22 | 3.00 | 1.88 | 0.76 | 2.74 | 57.39 | ||
| Nereis pulsatoria | 3.11 | 1.00 | 1.62 | 1.18 | 2.35 | 59.74 | ||
| Amphicorina rovignensis | 0.00 | 3.33 | 1.46 | 0.41 | 2.13 | 61.87 | ||
| Nereis rava | 0.78 | 2.67 | 1.46 | 1.30 | 2.13 | 64.00 | ||
| Ceratonereis (Composetia) costae | 1.11 | 2.00 | 1.42 | 0.92 | 2.06 | 66.07 | ||
| Odontosyllis ctenostoma | 0.33 | 2.44 | 1.36 | 0.86 | 1.98 | 68.05 | ||
| Lysidice unicornis | 2.33 | 0.44 | 1.14 | 1.11 | 1.66 | 69.71 | ||
| Syllis armillaris | 1.00 | 1.89 | 1.11 | 0.98 | 1.62 | 71.33 | ||
| Group 1.5 | Group 25 | |||||||
| 1.5 and 25 | Syllis armillaris | 1.00 | 13.22 | 9.06 | 1.75 | 10.24 | 10.24 | |
| 88.42% | Amphiglena mediterranea | 9.67 | 0.33 | 7.29 | 1.53 | 8.24 | 18.48 | |
| Nereis usticensis | 11.00 | 0.00 | 7.20 | 0.70 | 8.14 | 26.63 | ||
| Sphaerosyllis pirifera | 4.67 | 8.78 | 6.06 | 1.13 | 6.85 | 33.48 | ||
| Syllis rosea | 7.00 | 0.00 | 5.26 | 0.61 | 5.95 | 39.43 | ||
| Syllis gracilis | 0.22 | 6.67 | 5.09 | 1.73 | 5.75 | 45.18 | ||
| Platynereis dumerilii | 6.11 | 0.44 | 4.62 | 1.26 | 5.23 | 50.41 | ||
| Syllis prolifera | 5.44 | 1.00 | 3.82 | 0.86 | 4.32 | 54.73 | ||
| Syllis gerlachi | 0.22 | 4.11 | 2.87 | 1.31 | 3.24 | 57.97 | ||
| Hypsicomus stichophthalmos | 0.11 | 3.11 | 2.47 | 0.43 | 2.79 | 60.76 | ||
| Nereis pulsatoria | 3.11 | 0.11 | 2.45 | 1.26 | 2.77 | 63.54 | ||
| Exogone dispar | 2.89 | 0.11 | 2.34 | 0.99 | 2.65 | 66.18 | ||
| Lysidice unicornis | 2.33 | 0.11 | 1.62 | 1.25 | 1.83 | 68.01 | ||
| Nereididae juv. indet. | 2.00 | 0.11 | 1.43 | 0.74 | 1.62 | 69.63 | ||
| Dodecaceria concharum | 1.67 | 0.22 | 1.30 | 0.98 | 1.47 | 71.10 | ||
| Group 5 | Group 25 | |||||||
| 5 and 25 | Amphiglena mediterranea | 17.56 | 0.33 | 11.40 | 1.52 | 14.33 | 14.33 | |
| 79.59% | Syllis armillaris | 1.89 | 13.22 | 7.94 | 1.62 | 9.97 | 24.30 | |
| Sphaerosyllis pirifera | 5.67 | 8.78 | 5.07 | 1.23 | 6.37 | 30.66 | ||
| Syllis gracilis | 0.67 | 6.67 | 4.29 | 1.65 | 5.40 | 36.06 | ||
| Syllis prolifera | 5.33 | 1.00 | 3.24 | 1.13 | 4.08 | 40.14 | ||
| Nereis sp. 1 | 4.56 | 0.56 | 2.89 | 1.26 | 3.63 | 43.77 | ||
| Nereididae juv. indet. | 4.00 | 0.11 | 2.73 | 1.57 | 3.43 | 47.20 | ||
| Syllis gerlachi | 3.00 | 4.11 | 2.64 | 1.11 | 3.32 | 50.52 | ||
| Exogone dispar | 3.67 | 0.11 | 2.59 | 1.38 | 3.25 | 53.77 | ||
| Platynereis dumerilii | 3.56 | 0.44 | 2.46 | 1.12 | 3.09 | 56.86 | ||
| Hypsicomus stichophthalmos | 0.00 | 3.11 | 2.17 | 0.41 | 2.72 | 59.59 | ||
| Syllis variegata | 3.33 | 1.56 | 1.89 | 1.17 | 2.38 | 61.96 | ||
| Syllis corallicola | 3.11 | 0.44 | 1.75 | 1.43 | 2.20 | 64.17 | ||
| Amphicorina rovignensis | 3.33 | 0.22 | 1.67 | 0.44 | 2.10 | 66.26 | ||
| Odontosyllis ctenostoma | 2.44 | 0.00 | 1.56 | 0.85 | 1.96 | 68.22 | ||
| Nereis rava | 2.67 | 1.11 | 1.55 | 1.16 | 1.95 | 70.17 |
| Variable | SS(trace) | Pseudo-F | P | Prop. | Cumul. | |
|---|---|---|---|---|---|---|
| Depth | 20617 | 9.9394 | 0.0001 | 0.28448 | 0.28448 | |
| A | Peyssonnelia rubra | 5681.7 | 2.9532 | 0.0005 | 0.078398 | 0.36287 |
| Haliptilon sp. | 5214.1 | 2.9279 | 0.0008 | 0.071947 | 0.43482 | |
| Filamentous sp. 1 | 4161.5 | 2.488 | 0.001 | 0.057423 | 0.49224 | |
| Padina pavonica | 2822.7 | 1.7447 | 0.0263 | 0.038949 | 0.53119 | |
| Codium efusum | 2954.4 | 1.9048 | 0.0208 | 0.040766 | 0.57196 | |
| Depth | 20617 | 9.9394 | 0.0001 | 0.28448 | 0.28448 | |
| B | Encrusting | 5292.2 | 2.7277 | 0.0007 | 0.073024 | 0.3575 |
| Foliose | 4853.6 | 2.6764 | 0.0011 | 0.066971 | 0.42447 | |
| Articulated calcareous | 3064.3 | 1.7444 | 0.0306 | 0.042282 | 0.46675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikac, B.; Licciano, M.; Jaklin, A.; Iveša, L.; Giangrande, A.; Musco, L. Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean). Diversity 2020, 12, 408. https://doi.org/10.3390/d12100408
Mikac B, Licciano M, Jaklin A, Iveša L, Giangrande A, Musco L. Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean). Diversity. 2020; 12(10):408. https://doi.org/10.3390/d12100408
Chicago/Turabian StyleMikac, Barbara, Margherita Licciano, Andrej Jaklin, Ljiljana Iveša, Adriana Giangrande, and Luigi Musco. 2020. "Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean)" Diversity 12, no. 10: 408. https://doi.org/10.3390/d12100408
APA StyleMikac, B., Licciano, M., Jaklin, A., Iveša, L., Giangrande, A., & Musco, L. (2020). Diversity and Distribution Patterns of Hard Bottom Polychaete Assemblages in the North Adriatic Sea (Mediterranean). Diversity, 12(10), 408. https://doi.org/10.3390/d12100408