A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations
Abstract
:1. An Introduction to Pea Crabs
2. Studying Pea Crab Morphology
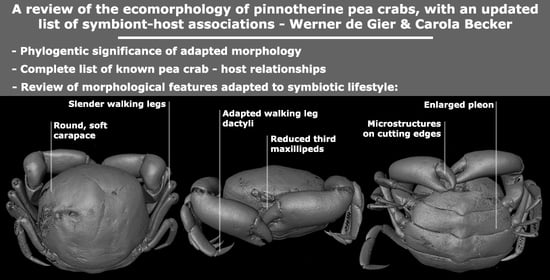
3. Adaptations in Pinnotherine Morphology
3.1. Carapace Shape, Size, and Ornamentation

3.2. Frontal Appendages and Mouthparts
3.3. Cheliped Morphology
3.4. Ambulatory Leg Adaptations
3.5. Sexual Anatomy and Larval Characters
3.6. Updated List of Symbiont-Host Associations
4. Phylogenetic Significance of Adaptive Features and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baeza, J.A. Crustaceans as symbionts: An overview of their diversity, host use, and lifestyles. In Lifestyles and Feeding Biology—The Natural History of the Crustacea; Thiel, M., Watling, L., Eds.; Oxford University Press: Oxford, UK, 2015; Volume 2, pp. 163–189. [Google Scholar]
- Castro, P. Symbiotic Brachyura. In Treatise on Zoology—Anatomy, Taxonomy, Biology, The Crustacea; Part C-I-Decapoda: Brachyura (Part-1); Castro, P., Davie, P.J.F., Guinot, D., Schram, F.P., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2015; Volume 9, pp. 543–581. [Google Scholar]
- Ng, P.K.L.; Guinot, D.; Davie, P.J.F. Systema Brachyurorum: Part I. An annotated checklist of extant Brachyuran crabs of the world. Raffles Bull. Zool. 2008, 17, 1–286. [Google Scholar]
- WoRMS: World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 22 June 2020).
- Palacios Theil, E.; Cuesta, J.A.; Felder, D.L. Molecular evidence for non-monophyly of the pinnotheroid crabs (Crustacea: Brachyura: Pinnotheroidea), warranting taxonomic reappraisal. Invertebr. Syst. 2016, 30, 1–27. [Google Scholar] [CrossRef]
- Schmitt, W.L.; McCain, J.C.; Davidson, E.S. Crustaceorum Catalogus: Decapoda I-Brachyura I-Fam. Pinnotheridae; Dr. W. Junk B.V.: Den Haag, The Netherlands, 1973; pp. 1–160. [Google Scholar]
- Manning, R.B. West African pinnotherid crabs, subfamily Pinnotherinae (Crustacea, Decapoda, Brachyura). Bull. Mus. Natl. D’histoire Nat. Paris Ser. 1993, 15, 125–177. [Google Scholar]
- Campos, E. Tumidotheres, a new genus for (Pinnotheres margarita) Smith, 1869, and Pinnotheres maculatus Say, 1818 (Brachyura: Pinnotheridae). J. Crustacean Biol. 1989, 9, 672–679. [Google Scholar] [CrossRef]
- Ocampo, E.H.; Spivak, E.D.; Baeza, J.A.; Luppi, T.A. Ontogenetic changes in the external anatomy of the parasitic castrator crab Calyptraeotheres garthi: Implications for the timing of host colonization and sexual behaviour. Biol. J. Linn. Soc. 2017, 120, 54–74. [Google Scholar]
- Derby, C.D.; Atema, J. Induced host odor attraction in the pea crab Pinnotheres maculatus. Biol. Bull. 1980, 158, 26–33. [Google Scholar] [CrossRef]
- McDermott, J.J. Hypersymbioses in the pinnotherid crabs (Decapoda: Brachyura: Pinnotheridae): A review. J. Nat. Hist. 2009, 43, 785–805. [Google Scholar] [CrossRef]
- Ahyong, S.T. Revision of Ostracotheres H. Milne Edwards, 1853 (Crustacea: Brachyura: Pinnotheridae). Raffles Bull. Zool. 2018, 66, 538–571. [Google Scholar]
- Werding, B.; Sanchez, H. Pinnotherid crabs of the genus Dissodactylus Smith, 1870, associated with irregular sea urchins at the Caribbean coast of Colombia (Crustacea: Decapoda: Pinnotheridae). Zool. Meded. 1989, 63, 35–42. [Google Scholar]
- Serène, R. A note on the systematics of the Brachyura and the morphology of commensal species. Proc. Ninth Pac. Sci. Congr. 1961, 10, 32–33. [Google Scholar]
- Becker, C.; Türkay, M. Host specificity and feeding in European pea crabs (Brachyura: Pinnotheridae). Crustaceana 2017, 90, 819–844. [Google Scholar] [CrossRef]
- Campos, E. Partial revision of the genus Fabia Dana, 1851 (Crustacea: Brachyura: Pinnotheridae). J. Nat. Hist. 1996, 30, 1157–1178. [Google Scholar] [CrossRef]
- Watanabe, T.; Henmi, Y. Morphological development of the commensal pea crab (Arcotheres sp.) in the laboratory reared specimens. J. Crustacean Biol. 2009, 29, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Palacios Theil, E.; Felder, D.L. Phylogeny of the genus Austinixa Heard & Manning, 1997, inferred from mitochondrial and nuclear molecular markers, with descriptions of three new species and redescription of Austinixa felipensis (Glassell, 1935) (Decapoda: Brachyura: Pinnotheridae). Zootaxa 2020, 4778, 101–134. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Ng, P.K.L. The pinnotherid type material of Semper (1880), Nauck (1880) and Bürger (1895) (Crustacea: Decapoda: Brachyura). Raffles Bull. Zool. Suppl. 2007, 191–226. [Google Scholar]
- Rathbun, M.J. The Grapsoid Crabs of America. Bull. U. S. Natl. Mus. 1918, 97, 1–461. [Google Scholar] [CrossRef]
- FEI Avizo ® 9.5.0 User Manual; Konrad-Zuse-Zentrum für Informationstechnik Berlin (ZIB): Berlin, Germany, 2018.
- Baeza, J.A.; Thiel, M. The mating system of symbiotic crustaceans: A conceptual model based on optimality and ecological constraints. In Evolutionary Ecology of Social and Sexual Systems: Crustaceans as Model Organisms; Duffy, J.E., Thiel, M., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 250–267. [Google Scholar]
- Christensen, A.M.; McDermott, J.J. Life-history and biology of the oyster crab, Pinnotheres ostreum Say. Biol. Bull. 1958, 114, 146–179. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Hamaishi, A. Mate acquisition by a solitary crab Zebrida adamsii, a symbiont of the sea urchin. J. Ethol. 1986, 4, 153–162. [Google Scholar] [CrossRef]
- Alves, F.D.R.; Hirose, G.L.; Barros-Alves, S.D.P.; Baeza, J.A. The mating system of the symbiotic pea‑crab Dissodactylus crinitichelis (Brachyura, Pinnotheridae): Monogamy or promiscuity? Mar. Biol. 2017, 164, 200. [Google Scholar] [CrossRef]
- Soong, K. Some life history observations on the pea crab, Pinnotheres tsingtaoensis, symbiotic with the bivalve mollusk, Sanguinolaria acuta. Crustaceana 1997, 70, 855–866. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Ng, P.K.L. Review of Durckheimia and Xanthasia, with descriptions of two new genera (Decapoda: Brachyura: Pinnotheridae). J. Crustacean Biol. 2005, 25, 116–129. [Google Scholar] [CrossRef] [Green Version]
- Ahyong, S.T.; Ng, P.K.L. Alain raymondi, a new species of deepwater pinnotherid crab (Crustacea: Decapoda: Brachyura) from the Philippines, commensal with holothurians. Zootaxa 2008, 1919, 61–68. [Google Scholar] [CrossRef]
- Ng, P.K.L. On the identities of Pinnotheres villosissimus Doflein, 1904, P. dofleini Lenz, in Lenz & Strunck, 1914, and P. pilumnoides Nobili, 1906 (Decapoda, Brachyura, Pinnotheridae) from the Western Indian Ocean. Crustaceana 2018, 91, 611–633. [Google Scholar] [CrossRef]
- Campos, E. The Pinnotheridae of the northeastern Pacific (Alaska to Mexico): Zoogeographical remarks and new bivalve hosts (Crustacea, Brachyura, Pinnotheridae). Zootaxa 2016, 4170, 311–329. [Google Scholar] [CrossRef] [Green Version]
- Manning, R.B.; Felder, D.L. Nannotheres moorei, a new genus and species of minute pinnotherid crab from Belize, Caribbean Sea (Crustacea: Decapoda: Pinnotheridae). Proc. Biol. Soc. Wash. 1996, 109, 311–317. [Google Scholar]
- Ahyong, S.T.; Ng, P.K.L. New species of pinnotherid crabs from Southeast Asia and Papua New Guinea (Crustacea: Decapoda: Brachyura). Zootaxa 2020, 4816, 333–349. [Google Scholar] [CrossRef]
- Cuesta, J.A.; Raso, J.E.G.; Abelló, P.; Marco-Herrero, E.; Silva, L.; Drake, P. A new species of pea crab from south-western Europe (Crustacea, Decapoda, Brachyura): Species description, geographic distribution and population structure with an identification key to European Pinnotheridae. J. Mar. Biol. Assoc. UK 2019, 99, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Hines, A.H. Constraint on reproductive output in brachyuran crabs: Pinnotherids test the rule. Am. Zool. 1992, 32, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Haines, C.M.C.; Edmunds, M.; Pewsey, A.R. The pea crab, Pinnotheres pisum (Linnaeus 1767), and its association with the common mussel, Mytilus edulis (Linnaeus, 1758), in the Solent (U.K.). J. Shellfish Res. 1994, 13, 5–10. [Google Scholar]
- Hsueh, P.W. Responses of the pea crab Pinnotheres taichungae to the life history patterns of its primary bivalve host Laternula marilina. J. Nat. Hist. 2003, 37, 1453–1462. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Galimany, E.; Abelló, P.; Cuesta, J.A.; Drake, P.; Ramón, M. Updating hosts and distribution range of the pea crab Pinnotheres bicristatus (Brachyura: Pinnotheridae). Mediterr. Mar. Sci. 2020, 21, 499–505. [Google Scholar] [CrossRef]
- Drake, P.; Marco-herrero, E.; Subida, M.D.; Arias, A.M.; Cuesta, J.A. Host use pattern of the pea crab Afropinnotheres monodi: Potential effects on its reproductive success and geographical expansion. Mar. Ecol. Prog. 2014, 498, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Sun, S.; Yuqi, W.; Baowen, Y.; Weibo, S. The prevalence of the pea crab, Pinnotheres sinensis, and its impact on the condition of the cultured mussel, Mytilus galloprovincialis, in Jiaonan waters (Shandong Province, China). Aquaculture 2006, 253, 57–63. [Google Scholar] [CrossRef]
- Salas-Moya, C.; Mena, S.; Wehrtmann, I.S. Reproductive traits of the symbiotic pea crab Austinotheres angelicus (Crustacea, Pinnotheridae) living in Saccostrea palmula (Bivalvia, Ostreidae), Pacific coast of Costa Rica. ZooKeys 2014, 457, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, E.H.; Nuñez, J.D.; Cledón, M.; Baeza, J.A. Host specific reproductive benefits, host selection behavior and host use pattern of the pinnotherid crab Calyptraeotheres garthi. J. Exp. Mar. Biol. Ecol. 2012, 429, 36–46. [Google Scholar] [CrossRef]
- Atkins, D. The moulting stages of the pea-crab (Pinnotheres pisum). J. Mar. Biol. Assoc. 1926, 14, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.; Türkay, M. Taxonomy and morphology of European pea crabs (Crustacea: Brachyura: Pinnotheridae). J. Nat. Hist. 2010, 44, 1555–1575. [Google Scholar] [CrossRef]
- Hoeksema, B.W. Het erwtenkrabbetje Pinnotheres pisum (L.), als commensaal van de paardemossel Modiolus modiolus (L.). Zeepaard 1981, 41, 79. [Google Scholar]
- Pearce, J.B. The Biology of the mussel crab, Fabia subquadrata, from the Waters of the San Juan Archipelago, Washington. Pac. Sci. 1966, 20, 3–35. [Google Scholar]
- Takahashi, T.; Otani, T.; Matsuura, S. Swimming behaviour of the pinnotherid, crab, Tritodynamia horvathi observed during the low temperature season. J. Mar. Biol. Assoc. UK 1999, 79, 375–377. [Google Scholar] [CrossRef]
- De Bruyn, C. Modalités Fonctionnelles et Évolutives des Parasitoses Développées par les Crabes Pinnotheridae aux Dépens des Échinides Fouisseurs. Ph.D. Thesis, Universite de Bourgogne, Bourgogne, France, 2010. [Google Scholar]
- Pohle, G.; Telford, M. Post-larval growth of Dissodactylus primitivus Bouvier, 1917 (Brachyura: Pinnotheridae) under Laboratory Conditions. Biol. Bull. 1982, 163, 211–224. [Google Scholar] [CrossRef]
- Komai, T.; Kei, K.; Ng, P.K.L. On the identity of the poorly known pea crab, Pinnothera obesa Dana, 1852, and description of a new species of Arcotheres Manning, 1993 from the Southwest Pacific (Decapoda: Brachyura: Pinnotheridae). Zootaxa 2020, 4822, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Zmarzly, D.L. Taxonomic review of pea crabs in the genus Pinnixa (Decapoda: Brachyura: Pinnotheridae) occurring on the California Shelf, with descriptions of two new species. J. Crustacean Biol. 1992, 12, 677–713. [Google Scholar] [CrossRef]
- Manning, R.B.; Felder, D.L. The Pinnixa cristata complex in the Western Atlantic, with descriptions of two new species (Crustacea: Decapoda: Pinnotheridae). Smithson. Contrib. Zool. 1989, 473, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.K.L.; Meyer, C. A new species of pea crab of the genus Serenotheres Ahyong & Ng, 2005 (Crustacea, Brachyura, Pinnotheridae) from the date mussel Leiosolenus Carpenter, 1857 (Mollusca, Bivalvia, Mytilidae, Lithophaginae) from the Solomon Islands. ZooKeys 2016, 623, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Ahyong, S.T.; Brown, D.E. Description of Durckheimia lochi n. sp., with an annotated check-list of Australian Pinnotheridae (Crustacea: Decapoda: Brachyura). Zootaxa 2003, 254, 1–20. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Ng, P.K.L. Visayeres acron, a new genus and species of pinnotherid crab (Crustacea: Decapoda: Brachyura) from the Philippines. Raffles Bull. Zool. Suppl. 2007, 16, 187–189. [Google Scholar]
- Holthuis, L.B. Limotheres, a new genus of pinnotherid crab, commensal of the bivalve Lima, from the Caribbean Sea. Zool. Meded. 1975, 48, 291–295. [Google Scholar]
- Guinot, D.; Wicksten, M.K. Camouflage: Carrying behaviour, decoration behaviour, and other modalities of concealmens in Brachyura. In Treatise on Zoology—Anatomy, Taxonomy, Biology, The Crustacea; Part C-I-Decapoda: Brachyura (Part-1); Castro, P., Davie, P.J.F., Guinot, D., Schram, F.P., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2015; Volume 9, pp. 583–638. [Google Scholar]
- Fransen, C.H.J.M. Shrimps and Molluscs/Garnalen en Weekdieren. Vita Mar. 1994, 42, 105–113. [Google Scholar]
- Fransen, C.H.J.M. Taxonomy, phylogeny, historical biogeography, and historical ecology of the genus Pontonia Latreille (Crustacea: Decapoda: Caridea: Palaemonidae). Zool. Verh. 2002, 336, 1–433. [Google Scholar] [CrossRef]
- Mebs, D. Chemical biology of the mutualistic relationships of sea anemones with fish and crustaceans. Toxicon 2009, 54, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Ohtsuka, S. A new species of the genus Abyssotheres (Crustacea, Decapoda, Brachyura, Pinnotheridae) from the Ryukyu Islands, Southwestern Japan, with taxonomic notes on the genus. Bull. Natl. Mus. Nat. Sci. Ser. A Zool. 2009, 35, 73–81. [Google Scholar]
- Campos, E. A new species and two new genera of pinnotherid crabs from the northeastern Pacific Ocean, with a reappraisal of the subfamily Pinnotherinae de Haan, 1833 (Crustacea: Brachyura: Pinnotheridae). Zootaxa 2009, 2022, 29–44. [Google Scholar] [CrossRef]
- Humann, P.; Deloach, N. Reef Creature Identification: Tropical Pacific, 1st ed.; New World Publications, Inc.: Jacksonville, FL, USA, 2017; pp. 1–497. [Google Scholar]
- Caro, T. The functional significance of coloration in crabs. Biol. J. Linn. Soc. 2018, 124, 1–10. [Google Scholar] [CrossRef]
- Marine Biodiversity Survey of St. Eustatius, Dutch Caribbean, 2015-Preliminary Results of the Statia Marine Biodiversity Expedition, 2015; Hoeksema, B.W. (Ed.) Naturalis Biodiversity Center, Leiden, and ANEMOON Foundation: Bennebroek, The Netherlands, 2016; pp. 1–157. [Google Scholar]
- Campos, E.; Peláez-zárate, V.A.; Solís-marín, F.A. Rediscovery, hosts and systematics of Holothuriophilus trapeziformis Nauck, 1880 (Crustacea, Brachyura, Pinnotheridae). Zootaxa 2012, 3528, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Atkins, D. On nocturnal colour change in the pea-crab (Pinnotheres veterum). Nature 1926, 117, 415–416. [Google Scholar] [CrossRef]
- De Bruyn, C.; David, B.; Motreuil, S.; Caulier, G.; Jossart, Q.; Rigaud, T.; De Ridder, C. Should I stay or should I go? Causes and dynamics of host desertion by a parasitic crab living on echinoids. Mar. Ecol. Prog. Ser. 2016, 546, 163–171. [Google Scholar] [CrossRef]
- Humann, P.; Deloach, N.; Wilk, L. Reef Creature Identification: Florida, Caribbean, Bahamas, 3rd ed.; New World Publications, Inc.: Jacksonville, FL, USA, 2013; pp. 1–295. [Google Scholar]
- Hopkins, T.S.; Scanland, T.B. The host relations of a pinnotherid crab, Opisthopus transversus Rathbun (Crustacea: Decapoda). Bull. South. Calif. Acad. Sci. 1964, 63, 175–180. [Google Scholar]
- Ng, P.K.L.; Manning, R.B. On two new genera of pea crabs parasitic in holothurians (Crustacea: Decapoda: Brachyura: Pinnotheridae) from the Indo-West Pacific, with notes on allied genera. Proc. Biol. Soc. Wash. 2003, 116, 901–919. [Google Scholar]
- Ng, P.K.L.; Ahyong, S.T.; Campos, E. Two new genera of pinnotherid crabs (Crustacea: Brachyura: Pinnotheroidea) from the Americas and the Western Pacific. Raffles Bull. Zool. 2019, 67, 337–351. [Google Scholar] [CrossRef]
- Miers, E.J. The Voyage of H.M.S. Challenger-Report of the Brachyura collected by H.M.S. Challenger during the years 1873–76. In Report on the Scientific Results of the Voyage of H.M.S.; Eyre & Spottiswoode: London, UK, 1886; pp. 1–362. [Google Scholar]
- De Man, J.G. Report on the podophthalmous Crustacea of the Mergui Archipelago, collected for the Trustees of the Indian Museum, Calcutta, by Dr. John Anderson–Part II. J. Linn. Soc. 1887, 22, 65–128. [Google Scholar] [CrossRef]
- Tai, A.; Yang, S. Crabs of the China Seas; China Ocean Press: Beijing, China; Springer: Berlin, Germany, 1991; pp. 421–437. [Google Scholar]
- Kruczynski, W.L. Relationship between depth and occurrence of pea crabs, Pinnotheres maculatus, in blue mussels, Mytilus edulis, in the Vicinity of Woods Hole, Massachusetts. Chesap. Sci. 1974, 15, 167–169. [Google Scholar] [CrossRef]
- Wass, M.L. A new pinnixid commensal with a holothurian (Crustacea: Decapoda). Tulane Stud. Zool. 1968, 14, 137–139. [Google Scholar] [CrossRef]
- Luckenbach, M.W.; Orth, R.J. A chemical defense in Crustacea? J. Exp. Mar. Biol. Ecol. 1990, 137, 79–87. [Google Scholar] [CrossRef]
- Jossart, Q.; Terrana, L.; De Ridder, C.; Eeckhaut, I.; Monteyne, D.; Caulier, G. To see or to smell: The role of vision in host-recognition by an ectoparasitic crab. Symbiosis 2020, 80, 97–101. [Google Scholar] [CrossRef]
- De Bruyn, C.; David, B.; De Ridder, C.; Rigaud, T. Asymmetric exploitation of two echinoid host species by a parasitic pea crab and its consequences for the parasitic life cycle. Mar. Ecol. Prog. Ser. 2010, 398, 183–191. [Google Scholar] [CrossRef]
- Dobson, N.C.; De Grave, S.; Johnson, M.L. Linking eye design with host symbiont relationships in pontoniine shrimps (Crustacea, Decapoda, Palaemonidae). PLoS ONE 2014, 9, e99505. [Google Scholar] [CrossRef] [Green Version]
- Dobson, N.C.; Johnson, M.L.; De Grave, S. Insights into the morphology of symbiotic shrimp eyes (Crustacea, Decapoda, Palaemonidae); the effects of habitat demands. PeerJ 2016, 4, e1926. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.; Barroso, D.; Hirose, G.L. Chemical recognition in the symbiotic pea crab Dissodactylus crinitichelis (Crustacea: Decapoda: Pinnotheridae): Host and conspecific cues. J. Exp. Mar. Biol. Ecol. 2018, 511, 108–112. [Google Scholar] [CrossRef]
- Davie, P.J.F.; Guinot, D.; Ng, P.K.L. Anatomy and functional morphology of Brachyura. In Treatise on Zoology–Anatomy, Taxonomy, Biology, The Crustacea; Part C—Decapoda: Brachyura (Part-1); Castro, P., Davie, P.J.F., Guinot, D., Schram, F.P., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2015; Volume 9, pp. 11–163. [Google Scholar]
- Campos, E. Two new genera of pinnotherid crabs from the tropical Eastern Pacific (Decapoda: Brachyura: Pinnotheridae). J. Crustacean Biol. 2002, 22, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.; Hernández-Aguilera, J.L. First record and range extension of the Jewel Box clam crab Gemmotheres chamae (Roberts, 1975) to the Gulf of Mexico, with comments on the systematics of the pinnotherines with a 2-segmented palp on the third maxilliped (Crustacea: Brachyura: Pinnotherida. Naupl. -J. Braz. Crustacean Soc. 2020, 28, 1–7. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Ng, P.K.L. Aphanodactylidae, a new family of thoracotreme crabs (Crustacea: Brachyura) symbiotic with polychaete worms. Zootaxa 2009, 2289, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Takeda, M.; Masahito, P. Systematic notes on the pinnotherid crabs of the genus Pinnaxodes (Crustacea: Decapoda: Brachyura). Bull. Natl. Sci. Mus. Tokyo 2000, 26, 99–112. [Google Scholar]
- Campos, E. Partial revision of pinnotherid crab genera with a two-segmented palp on the third maxilliped (Decapoda: Brachyura). J. Crustacean Biol. 1996, 16, 556–563. [Google Scholar] [CrossRef]
- Campos, E. Calyptraeotheres, a new genus of Pinnotheridae for the limpet crab Fabia granti Glassell, 1933 (Crustacea, Brachyura). Proc. Biol. Soc. Wash. 1990, 103, 364–371. [Google Scholar]
- Wells, H.W.; Wells, M.J. Observations on Pinnaxodes floridensis, a new species of pinnotherid crustacean commensal in holothurians. Bull. Mar. Sci. Gulf Caribb. 1961, 11, 267–279. [Google Scholar]
- Ng, P.K.L.; Kumar, A.B. A new species of Afropinnotheres Manning, 1993 (Crustacea, Brachyura, Pinnotheridae) from southwestern India, the first record of the genus from the Indian Ocean, with a review of the Pinnotheridae of India and adjacent seas. Zootaxa 2015, 3947, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Griffith, H. Phylogenetic relationships and evolution in the genus Dissodactylus Smith, 1870 (Crustacea: Brachyura: Pinnotheridae). Can. J. Zool. 1987, 65, 2292–2310. [Google Scholar] [CrossRef]
- Griffith, H. Taxonomy of the genus Dissodactylus (Crustacea: Brachyura: Pinnotheridae) with descriptions of three new species. Bull. Mar. Sci. 1987, 40, 397–422. [Google Scholar]
- Pohle, G. Structure, function, and development of setae on gill-grooming appendages and associated mouthparts of pinnotherid crabs (Decapoda: Brachyura). Can. J. Zool. 1989, 67, 1690–1707. [Google Scholar] [CrossRef]
- Pohle, G.; Marques, F. Phylogeny of the Pinnotheridae: Larval and adult evidence, with emphasis on Phylogeny of the Pinnotheridae: Larval and adult evidence, with emphasis on the evolution of gills. Invertebr. Reprod. Dev. 1998, 33, 229–239. [Google Scholar] [CrossRef]
- Vader, W. Associations between amphipods (Crustacea: Amphipoda) and sea anemones (Anthozoa: Actiniaria). Aust. Mus. Mem. 1983, 18, 141–153. [Google Scholar] [CrossRef]
- Telford, M. Echinoderm spine structure, feeding and host relationships of four species of Dissodactylus (Brachyura: Pinnotheridae). Bull. Mar. Sci. 1982, 32, 584–594. [Google Scholar]
- Palacios Theil, E.; Felder, D.L. Molecular phylogeography of Tumidotheres maculatus (Say, 1818) and Zaops ostreus (Say, 1817) (Crustacea: Decapoda: Pinnotheridae) in the western Atlantic, with description of a new species and synonymy of Epulotheres Manning, 1993. Mar. Biol. Res. 2019, 15, 548–567. [Google Scholar] [CrossRef]
- Jones, S.; Mahadevan, S. Notes on animal associations. 5. The pea crab Pinnotheres deccanensis Chopra inside the respiratory tree of the sea cucumber, Holothuria scabra Jager. J. Mar. Biol. Assoc. India 1965, 7, 377–380. [Google Scholar]
- Bell, J.L. Changing residence: Dynamics of the symbiotic relationship between Dissodactylus mellitae Rathbun (Pinnotheridae) and Mellita quinquiesperforata (Leske) (Echinodermata). J. Exp. Mar. Biol. Ecol. 1984, 82, 101–115. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Ngo, V.T. Solenotheres prolixus, a new genus and new species of pinnotherid crab (Crustacea: Decapoda: Brachyura) associated with the razor clam, Solen corneus Lamarck, 1818 (Solenidae) in Vietnam. Zootaxa 2010, 2570, 61–68. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Ho, P. A new genus for Fabia obtusidentata Dai, Feng, Song and Chen, 1980, a pea crab (Decapoda: Brachyura: Pinnotheridae) symbiotic with the moon scallop Amusium pleuronectes (Linnaeus, 1758) (Mollusca: Pectinidae). J. Crustacean Biol. 2016, 36, 740–751. [Google Scholar] [CrossRef] [Green Version]
- Manning, R.B. Viridotheres marionae, a new genus and species of pinnotherid crab from West Africa (Crustacea: Decapoda: Brachyura). Zool. Meded. 1996, 70, 271–273. [Google Scholar]
- Campos, E.; Manning, R.B. The Identities of Pinnotheres nudus Holmes, 1895 and P. nudus sensu Weymouth, 1910 (Crustacea: Decapoda: Pinnotheridae). Proc. Biol. Soc. Wash. 2000, 113, 799–805. [Google Scholar]
- Kazmi, Q.B.; Manning, R.B. A new genus and species of pinnotherid crab from Karachi, northern Arabian Sea (Crustacea, Decapoda, Brachyura). J. Nat. Hist. 2003, 37, 1085–1089. [Google Scholar] [CrossRef]
- Ahyong, S.T. Holotheres danielae, a new species of pinnotherid crab from the Indo-West Pacific (Decapoda, Brachyura), with a key to the genus. Crustaceana Monogr. 2010, 11, 35–40. [Google Scholar]
- Mesce, K.A. Morphological and physiological identification of chelar sensory structures in the hermit crab Pagurus hirsutiusculus (Decapoda). J. Crustacean Biol. 1993, 13, 95–110. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Swimming in the hard stage of the pea crab, Pinnotheres pisum (L.). J. Nat. Hist. 1972, 6, 475–480. [Google Scholar] [CrossRef]
- Stauber, L.A. Pinnotheres ostreum, parasitic on the American oyster, Ostrea (Gryphaea) virginica. Biol. Bull. 1945, 88, 269–291. [Google Scholar] [CrossRef]
- Hsueh, P.W.; Huang, J.F. A new record of Pinnotheres bidentatus Sakai, 1939 (Decapoda: Brachyura: Pinnotheridae), from Taiwan. Crustacean Res. 1996, 25, 54–58. [Google Scholar] [CrossRef]
- Atkins, D. British pea-crabs (Pinnotheres). Nature 1958, 4615, 1087. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Ho, P. Orthotheres baoyu, a new species of pea crab (Crustacea: Brachyura: Pinnotheridae) associated with abalones from Tungsha Island, Taiwan; with notes on the genus. Raffles Bull. Zool. 2016, 64, 229–241. [Google Scholar]
- Gordon, I. On a few Indo-Pacific species of Pinnotheres, with special reference to asymmetry of the walking legs. J. Linn. Soc. Lond. Zool. 1936, 40, 163–180. [Google Scholar] [CrossRef]
- Griffin, D.J.G.; Campbell, B.M. The sub-littoral Goneplacidae and Pinnotheridae (Crustacea: Brachyura) of Moreton Bay. Mem. Qld. Mus. 1969, 15, 141–163. [Google Scholar]
- Campos, E. A new crab species of the genus Arcotheres Manning, 1993, from Thailand (Crustacea, Brachyura, Pinnotheridae). Zoosystema 2001, 23, 493–497. [Google Scholar]
- Campos, E. Remarks on the sexual dimorphism and taxonomy of Fabia Dana, 1851 (Crustacea, Brachyura, Pinnotheridae). Zootaxa 2013, 3616, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.; Manning, R.B. Authorship and diagnosis of the genus Arcotheres Manning, 1993 (Crustacea: Brachyura: Pinnotheridae). Raffles Bull. Zool. 2001, 49, 167–170. [Google Scholar]
- Campos, E.; Griffith, H. Clypeasterophilus, a new genus to receive the small-palped species of the Dissodactylus complex (Brachyura: Pinnotheridae). J. Crustacean Biol. 1990, 10, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Manning, R.B.; Galil, B. A new genus of pinnotherid crab from the Indian Ocean (Crustacea: Decapoda: Brachyura). Proc. Biol. Soc. Wash. 2000, 113, 66–69. [Google Scholar]
- Hamel, J.; Ng, P.K.L.; Mercier, A. Life cycle of the pea crab Pinnotheres halingi sp. nov., an obligate symbiont of the sea cucumber Holothuria scabra Jaeger. Ophelia 1999, 50, 149–175. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Clark, P.F.; Mitra, S.; Kumar, A.B. Arcotheres borradailei (Nobili, 1906) and Pinnotheres ridgewayi Southwell, 1911: A reassessment of characters and generic assignment of species to Arcotheres Manning, 1993 (Decapoda, Brachyura, Pinnotheridae). Crustaceana 2017, 90, 1079–1097. [Google Scholar] [CrossRef]
- Trivedi, J.N.; Vachhrajani, K.D.; Ng, P.K.L. Redescription of Arcotheres placunae (Hornell & Southwell, 1909) (Crustacea: Decapoda: Brachyura: Pinnotheridae) from India and Pakistan. Zootaxa 2018, 4433, 50–58. [Google Scholar]
- Trivedi, J.N.; Gosavi, S.; Vachhrajani, K.D.; Mitra, S.; Ravinesh, R.; Ng, P.K.L. On the identities of Nepinnotheres vicajii (Chhapgar, 1957) and Arcotheres casta (Antony & Kuttyamma, 1971) from western India: Conspecificity and taxonomy (Decapoda, Brachyura, Pinnotheridae). Zootaxa 2020, 4809, 496–508. [Google Scholar]
- De Gier, W.; Fransen, C.H.J.M. Odontonia plurellicola sp. n. and Odontonia bagginsi sp. n., two new ascidian-associated shrimp from Ternate and Tidore, Indonesia, with a phylogenetic reconstruction of the genus (Crustacea, Decapoda, Palaemonidae). Zookeys 2018, 765, 123–160. [Google Scholar] [CrossRef]
- Campos, E. Inclusion of the austral species Pinnotheres politus (Smith, 1869) and Pinnotheres garthi Fenucci, 1975 within the genus Calyptraeotheres Campos, 1990 (Crustacea: Brachyura: Pinnotheridae). Proc. Biol. Soc. Wash. 1999, 112, 536–540. [Google Scholar]
- Hernández-Ávila, I.; Campos, E. Calyptraeotheres hernandezi (Crustacea: Brachyura: Pinnotheridae), a new crab symbiont of the West Indian cup-and-saucer Crucibulum auricula (Gmelin) (Mollusca: Gastropoda: Calyptraeidae) off Cubagua Island, Venezuela. Proc. Biol. Soc. Wash. 2006, 119, 43–48. [Google Scholar] [CrossRef]
- Campos, E.; Hernández-ávila, I. Phylogeny of Calyptraeotheres Campos, 1990 (Crustacea, Decapoda, Brachyura, Pinnotheridae) with the description of C. pepeluisi new species from the tropical Mexican Pacific. Zootaxa 2010, 2691, 41–52. [Google Scholar] [CrossRef]
- Ayón-parente, M.; Hendrickx, M.E. Calyptraeotheres sp. nov. (Crustacea: Decapoda: Pinnotheridae), symbiont of the slipper shell Crepidula striolata Menke, 1851 (Mollusca: Gastropoda: Calyptraeidae) from the Gulf of California, Mexico. Zootaxa 2014, 3872, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.H. Fecundity and reproductive output in nine species of Cancer crabs (Crustacea, Brachyura, Cancridae). Can. J. Fish. Aquat. Sci. 1991, 48, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Hartnoll, R.G. Reproductive Investment in Brachyura. Hydrobiologica 2006, 557, 31–40. [Google Scholar] [CrossRef]
- Telford, M. Distribution of two species of Dissodactylus (Brachyura Pinnotheridae) among their echinoid host populations in Barbados. Bull. Mar. Sci. 1978, 28, 651–658. [Google Scholar]
- Becker, C. European Pea Crabs-Taxonomy, Morphology, and Host-Ecology (Crustacea: Brachyura: Pinnotheridae). Ph.D. Thesis, Universität in Frankfurt am Main, Frankfurt, Germany, 2010. [Google Scholar]
- George, S.B.; Boone, S. The ectosymbiont crab Dissodactylus mellitae-Sand dollar Mellita isometra relationship. J. Exp. Mar. Biol. Ecol. 2003, 294, 235–255. [Google Scholar] [CrossRef]
- McClay, C.L.; Becker, C. Reproduction in Brachyura. In Treatise on Zoology—Anatomy, Taxonomy, Biology, The Crustacea; Part C-I-Decapoda: Brachyura (Part-1); Castro, P., Davie, P.J.F., Guinot, D., Schram, F.P., von Vaupel Klein, J.C., Eds.; Brill: Leiden, The Netherlands, 2015; Volume 9, pp. 185–243. [Google Scholar]
- Becker, C.; Brandis, D.; Storch, V. Morphology of the female reproductive system of European pea crabs (Crustacea, Decapoda, Brachyura, Pinnotheridae). J. Morphol. 2011, 272, 12–26. [Google Scholar] [CrossRef]
- Becker, C.; Klaus, S.; Tudge, C. Male internal reproductive structures of European pea crabs (Crustacea, Decapoda, Brachyura, Pinnotheridae): Vas deferens morphology and spermatozoal ultrastructure. J. Morphol. 2013, 274, 1312–1322. [Google Scholar] [CrossRef]
- Vehof, J.; van der Meij, S.E.T.; Türkay, M.; Becker, C. Female reproductive morphology of coral-inhabiting gall crabs (Crustacea: Decapoda: Brachyura: Cryptochiridae). Acta Zool. 2016, 97, 117–126. [Google Scholar] [CrossRef]
- de Souza, L.P.; Silva, J.R.F. Morfología del sistema reproductivo de las hembras del cangrejo rojo de mangle (Goniopsis cruentata Latreille, 1803). Sci. Mar. 2009, 73, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Cobo, V.J.; Fransozo, A. Physiological maturity and relationships of growth and reproduction in the red mangrove crab Goniopsis cruentata (Latreille) (Brachyura, Grapsidae) on the coast of São Paulo, Brazil. Rev. Bras. Zool. 2005, 22, 219–223. [Google Scholar] [CrossRef]
- Strathmann, R.R.; Strathmann, M.F. The relationship between adult size and brooding in marine invertebrates. Am. Nat. 1982, 119, 91–101. [Google Scholar] [CrossRef]
- Bush, A.O.; Fernández, J.C.; Esch, G.W.; Seed, J.R. Parasitism: The Diversity and Ecology of Animal Parasites; Cambridge University Press: Cambridge, UK, 2001; pp. 1–516. [Google Scholar]
- Martin, J.W. 55: Brachyura. In Atlas of Crustacean Larvae; Martin, J.W., Olesen, J., Høeg, J.T., Eds.; Johns Hopkins University Press: Baltimore, ML, USA, 2014; pp. 1–384. [Google Scholar]
- Gonzalez-Canales, M.E.; Marco-Herrero, E.; Andreu-Cazenave, M.; González-Gordillo, J.I. Larval development of the symbiotic pea crab Pinnaxodes chilensis (H. Milne Edwards, 1837) (Decapoda, Brachyura, Pinnotheridae) reared in laboratory. Arthropod Struct. Dev. 2018, 47, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pohle, G. Larval development of Dissodactylus Rugatus Bouvier, 1917 (= D. Calmani Rathbun, 1918) (Brachyura: Pinnotheridae) reared under laboratory conditions. J. Crustacean Biol. 1984, 4, 572–588. [Google Scholar] [CrossRef]
- Lebour, M.V. The Larval Stages of the Plymouth Brachyura. Proc. Zool. Soc. Lond. 1928, 98, 473–560. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Drake, P.; González-Gordillo, J.I.; Cuesta, J.A. Larval development of the pea crab Afropinnotheres monodi Manning, 1993 (Decapoda, Pinnotheridae) using plankton-collected and laboratory-reared specimens: Effects of temperature. Mar. Biol. Res. 2016, 12, 43–55. [Google Scholar] [CrossRef]
- Morgan, S.G. Morphological and behavioral antipredatory adaptations of decapod zoeae. Oecologia 1987, 73, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Goodbody, I. Abbreviated development in a pinnotherid crab. Nature 1960, 185, 704–705. [Google Scholar] [CrossRef]
- Bolaños, J.; Rivero, W.; Hernández, J.; Magán, I.; Hernández, G.; Cuesta, J.A.; Felder, D.L. Abbreviated larval development of the pea crab Orthotheres barbatus (Decapoda: Brachyura: Pinnotheridae) described from laboratory-reared material, with notes on larval characters of the Pinnotherinae. J. Crustacean Biol. 2005, 25, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Bolaños, J.; Cuesta, J.A.; Hernández, G.; Hernández, J.; Felder, D.L. Abbreviated larval development of Tunicotheres moseri (Rathbun, 1918) (Decapoda: Pinnotheridae), a rare case of parental care among brachyuran crabs*. Sci. Mar. 2004, 68, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Silas, E.G.; Alagarswami, K. On an instance of parasitisation by the pea-crab (Pinnotheres sp.) on the backwater clam [Meretrix casta (Chemnitz)] from India, with a review of the work on the systematics, ecology biology and ethology of pea crabs of the genus Pinnotheres Latreille*. In Proceedings of the Symposium on Crustacea, Ernakulam, India, 12–15 January 1965; Part III (Series 2). pp. 1161–1227. [Google Scholar]
- Pérez-Miguel, M.; Drake, P.; Garcia Raso, J.E.; Mamám Menéndez, L.; Navas, J.I.; Cuesta, J.A. European Pinnotheridae (Crustacea, Decapoda, Brachyura): Species, distribution, host use and DNA barcodes. Mar. Biodivers. 2019, 49, 57–68. [Google Scholar] [CrossRef]
- Manning, R.B. A new genus and species of pinnotherid crab from Karachi, northern Arabian Sea (Crustacea, Decapoda, Brachyura). Zoosystema 1998, 20, 357–362. [Google Scholar] [CrossRef]
- Devi, K.N.; Shyamasundari, K. A new species of Pinnotheres Latreille (Decapoda: Brachyura) from Visakhapatnam coast of Bay of Bengal, Andhra Pradesh. J. Bombay Nat. Hist. Soc. 1989, 86, 217–221. [Google Scholar]
- Kazmi, Q.; Sultana, R.; Ghory, F. Redescription of Arcotheres placunae and three new records, A. aff. alcocki, A. casta and Pinnotheres quadratus from Pakistan with a note on previously recorded Pakistani Pinnotherid crabs. Pak. J. Mar. Sci. 2016, 25, 131–143. [Google Scholar]
- Sakai, T. Studies on the Crabs of Japan IV; Yokendo: Tokyo, Japan, 1939; pp. 583–605. [Google Scholar]
- Ng, P.K.L. Arcotheres placunicola, a new species of pea crab (Crustacea: Brachyura: Pinnotheridae) from the window-pane shell, Placuna ephippium Philipsson, 1788 (Placunidae) in Singapore. Raffles Bull. Zool. 2018, 66, 474–485. [Google Scholar]
- Mohanty, B.; Raut, D.; Dev Roy, M.K.; Raman, A.V.; Patnaik, L.; Nayak, A.; Rout, S.S.; Dash, B. New host record of pea crab Arcotheres purpureus (Alcock, 1900) (Crustacea: Decapoda: Pinnotheridae) with first description of male. Mar. Biodivers. Rec. 2018, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Kuo, A.-L.; Lin, F.-J.; Hsu, H.-T.; Chan, Y.-S.; Ueng, Y.-T. The population structure and parasitic relationships of oyster (Crassostrea angulata), Arcotheres sinensis (Pinnotheridae), and Rhopalione sinensis (Bopyridae) at the oyster reefs of Western Taiwan. Crustaceana 2018, 91, 1433–1451. [Google Scholar] [CrossRef]
- Ribeiro, F.B.; Matthews-Cascon, H.; Bezerra, L.E.A. Record of the pea-crab Calyptraeotheres garthi (Fenucci, 1975) (Brachyura, Pinnotheridae) in Tropical Atlantic Ocean. Arq. Ciências Mar. 2020, 53, 143–148. [Google Scholar] [CrossRef]
- Filho, J.E.M.; Brito dos Santos, R.; Ribeiro, C.C. Host selection, host-use pattern and competition in Dissodactylus crinitichelis and Clypeasterophilus stebbingi (Brachyura: Pinnotheridae). Symbiosis 2014, 63, 99–110. [Google Scholar] [CrossRef]
- Campos, E.; Manning, R.B. Pinnotheres malaguena Garth, 1948, a new member of the genus Fabia Dana, 1851 (Crustacea: Brachyura: Pinnotheridae). Proc. Biol. Soc. Wash. 1998, 111, 912–915. [Google Scholar]
- Campos, E. Systematics and taxonomic remarks on Pinnotheres muliniarium Rathbun, 1918 (Crustacea: Brachyura: Pinnotheridae). Proc. Biol. Soc. Wash. 1993, 106, 92–101. [Google Scholar]
- Campos, E. Comments on taxonomy of the genus Orthotheres Sakai, 1969 (Crustacea, Brachyura, Pinnotheridae). Bull. Mar. Sci. 1989, 44, 1123–1128. [Google Scholar]
- Page, R.D.M. Description of a new species of Pinnotheres, and redescription of P. novaezelandiae (Brachyura: Pinnotheridae). N. Z. J. Zool. 1983, 10, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Ahyong, S.T. Resolution of the identity of Pinnotheres latipes Hombron & Jacquinot, 1846 and description of a new species of Viridotheres Manning, 1996 (Decapoda: Brachyura: Pinnotheridae): Two symbionts of bivalve molluscs. J. Crustacean Biol. 2020, 1–8. [Google Scholar] [CrossRef]
- Campos, E. Taxonomy of Pinnotheres bipunctatus Nicolet, 1849 with a distributional checklist of the Pinnotheridae of Chile and Peru, and a list of the Crustacea described by Hercule Nicolet in the atlas of the physical and political history of Chile. Lat. Am. J. Aquat. Res. 2017, 45, 379–390. [Google Scholar] [CrossRef]
- Marin, I.N. Finding of the pea crab Pinnaxodes mutuensis Sakai, 1939 (Crustacea: Decapoda: Pinnotheridae) in an unusual host in Busse Lagoon, southern Sakhalin. Russ. J. Mar. Biol. 2014, 40, 486–489. [Google Scholar] [CrossRef]
- De Melo, G.A.S.; Boehs, G. Rediscovery of Holothuriophilus tomentosus (Ortmann) comb. nov. (Crustacea, Brachyura, Pinnotheridae) in the Brazilian coast. Rev. Bras. Zool. 2004, 21, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, R.M.; Mackinnon, D.I.; Endo, K.; Chirino-Galvez, L. Pinnotheres laqueisakai (Decapoda: Pinnotheridae), a tiny crab commensal within the brachiopod Laqueus rubellus (Sowerby) (Terebratulida: Laqueidae). J. Paleontol. 1996, 70, 303–311. [Google Scholar] [CrossRef]
- Tai, A.; Song, W. Crabs of the China Seas; China Ocean Press: Beijing, China, 1991; pp. 54–62. (In Chinese) [Google Scholar]
- Ahyong, S.T. Discovery of Viridotheres Manning, 1996 in the southwestern Pacific and first record of Discorsotheres camposi Ahyong, 2018 from New Caledonia (Crustacea: Brachyura: Pinnotheridae). Zootaxa 2020, 4763, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Ahyong, S.T. First Indonesian Viridotheres Manning, 1996, and redescription of male Afropinnotheres dofleini Manning, 1993, from South Africa (Decapoda, Pinnotheridae). Crustaceana 2019, 92, 107–118. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Komai, T.; Watanabe, T. First Viridotheres Manning, 1996, from Japan, with a key to the species (Decapoda, Brachyura, Pinnotheridae). In Studies on Eumalacostraca: A Homage to Masatsune Takeda; Komatsu, H., Okuno, J., Fukuoka, K., Eds.; Brill: Leiden, The Netherlands, 2012; pp. 35–48. [Google Scholar] [CrossRef] [Green Version]
- Goto, R.; Ohsuga, K.; Kato, M. Mode of life of Anomiostrea coralliophila Habe, 1975 (Ostreidae): A symbiotic oyster living in ghost-shrimp burrows. J. Molluscan Stud. 2014, 80, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Števčić, Z.; Castro, P.; Gore, R.H. Re-establishment of the family Eumedonidae Dana, 1853 (Crustacea: Brachyura). J. Nat. Hist. 1988, 22, 1301–1324. [Google Scholar] [CrossRef] [Green Version]
- Ng, P.K.L.; Corbari, L. Crustacea: Crab Legacies. In Voyageurs, Explorateurs et Scientifiques. The French and Natural History in Singapore; First Printers; Low, M.E.Y., Pockington, K., Josuh, W.F.A., Eds.; Lee Kong Chian Natural History Museum: Singapore, 2019; pp. 234–253. [Google Scholar]
- Aikawa, H. On larval forms of some Brachyura, Paper II: A note on indeterminate zoeas. Rec. Oceanogr. Work. Jpn. 1933, 5, 124–154. [Google Scholar]
- Manning, R.B. Three genera removed from the synonymy of Pinnotheres Bosc, 1802 (Brachyura: Pinnotheridae). Proc. Biol. Soc. Wash. 1993, 106, 523–531. [Google Scholar]
- Tesch, J.J. Siboga Expeditie: The Decapoda Brachyura of the Siboga Expedition I-Hymenosomidae. Retroplumidae, Ocypodidae, Grapsidae and Gecarcinidae; E.J. Brill: Leiden, The Netherlands, 1918; pp. 1–148. [Google Scholar]
- Jiang, W.; Liu, R. New species and new records of pinnotherid crabs (Crustacea: Decapoda: Brachyura) from the Yellow Sea. Zool. Anz. 2011, 250, 488–496. [Google Scholar] [CrossRef]
- Sakai, T. The Crabs of Sagami Bay Collected by His Majesty the Emperor of Japan; Maruzen Co.: Tokyo, Japan, 1965; pp. 1–206. [Google Scholar]
- Green, T.M. Pinnaxodes gigas, a new species of pinnotherid crab from the Gulf of California (Decapoda: Brachyura: Pinnotheridae). Proc. Biol. Soc. Wash. 1992, 105, 775–779. [Google Scholar]
- Glassell, S.A. New or little known crabs from the Pacific coast of northern Mexico. Trans. San Diego Soc. Nat. Hist. 1935, 8, 91–106. [Google Scholar]
- Campos, E.; De Campos, A.R.; Ramirez, J. Remarks on distribution and hosts for symbiotic crustaceans of the Mexican Pacific (Decapoda and Isopoda). Proc. Biol. Soc. Wash. 1992, 105, 753–759. [Google Scholar]
- Sakai, K. On the occurrence of three species of crabs on Shikoku Island, Japan, and a new species, Pinnotheres taichungae nov. spec., from Taiwan (Decapoda, Brachyura). Crustaceana 2000, 73, 1155–1162. [Google Scholar] [CrossRef]
- Yamada, M.; Ishibashi, R.; Toyoda, K.; Kawamura, K.; Komaru, A. Phylogeography of the brackish water clam Corbicula japonica around the Japanese archipelago inferred from mitochondrial COII gene sequences. Zool. Sci. 2014, 31, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Nobili, M.G. Diagnoses préliminaires de 34 espèces et variétés nouvelles, et de 2 genres nouveaux de décapodes de la Mer Rouge. Bull. Muséum D’histoire Nat. Paris Prem. Séries 1906, 6, 393–411. [Google Scholar]
- Laurie, R.D. Reports on the marine biology of the Sudanese Red Sea. -XXI. On the Brachyura. J. Linn. Soc. Lond. Zool. 1915, 31, 407–475. [Google Scholar] [CrossRef] [Green Version]







| Species | Host | Host Species | Distr. | References |
|---|---|---|---|---|
| Abyssotheres1 | ||||
| A. abyssicola (Alcock & Anderson, 1899) | Bivalvia | Acesta indica (Smith, 1899) | IWP | [119] |
| A. acesticola Komatsu & Ohtsuka, 2009 | Bivalvia | Acesta philippinensis (Bartsch, 1913) | IWP | [60] |
| Afropinnotheres | ||||
| A. crosnieri Manning, 1993 | ? | ? | ATL | [7] |
| A. dofleini Lenz, 1914 1 | Bivalvia, Ascidia | Atrina squamifera (Sowerby, 1835), Perna perna (Linnaeus, 1758), Pinna sp.; Ascidia sydneiensis (Stimpson, 1855), Pyura stolonifera (Heller, 1878) | ATL/IWP | [29] |
| A. guinotae Manning, 1993 | Bivalvia | ‘unnamed bivalve mussel’ | ATL | [7] |
| A. larissae (Machkevskiy, 1992) | Bivalvia | Crassostrea tulipa (Lamarck, 1819) | ATL | [7] |
| A. monodi Manning, 1993 | Bivalvia | Cerastoderma edule (Linnaeus, 1758), Cerastoderma glaucum (Bruguière, 1789), Chamelea gallina (Linnaeus, 1758), Donax trunculus Linnaeus, 1758, Eastonia rugosa (Helbling, 1779), Mactra stultorum (Linnaeus, 1758), Mytilus galloprovincialis Lamarck, 1819, Polititapes aureus (Gmelin, 1791), Ruditapes decussatus (Linnaeus, 1758), Scrobicularia plana (da Costa, 1778), Spisula solida (Linnaeus, 1758), Venerupis corrugata (Gmelin, 1791) | ATL | [152] |
| A. ratnakara Ng & Kumar, 2015 | Bivalvia | Atrina vexillum (Born, 1778), Perna perna (Linnaeus, 1758) | IWP | [91,121] |
| Alain | ||||
| A. crosnier Manning, 1998 | Holothuria | Molpadia sp. | IWP | [153] |
| A. raymondi Ahyong & Ng, 2008 | Holothuria | ‘unidentified deep-water holothurian’ | IWP | [28] |
| Alainotheres | ||||
| A. leloeuffi (Crosnier, 1969) 2 | ? | ? | ATL | [7] |
| Amusiotheres | ||||
| A. hanumantharaoi Devi & Shyamasundari, 1989 | Bivalvia | Amusium pleuronectes (Linnaeus, 1758) | IWP | [154] |
| A. obtusidentatus (Tai et al., 1980) | Bivalvia | Amusium pleuronectes (Linnaeus, 1758), Ylistrum japonicum (Gmelin, 1791) | IWP | [102] |
| Arcotheres | ||||
| A. alcocki (Rathbun, 1909) | Bivalvia | Atrina vexillum (Born, 1778), Meretrix sp., Meretrix casta (Gmelin, 1791), Mytilus sp., Pinna atropurpurea (Sowerby, 1825), Tegillarca granosa (Linnaeus, 1758) | IWP | [6,155] |
| A. arcophilus (Bürger, 1895) | Bivalvia | Arca sp. | IWP | [6] |
| A. atrinae (T. Sakai, 1939) | Bivalvia | Atrina japonica (Reeve, 1858) | IWP | [156] |
| A. boninensis (Stimpson, 1858) | Bivalvia | Saccostrea echinata (Quoy & Gaimard, 1835) | IWP | [6] |
| A. borradailei (Nobili, 1906) | Bivalvia | Mya sp., Pinna sp. | IWP | [6] |
| A. coarctatus (Bürger, 1895) | Bivalvia | Polymesoda spp. | IWP | [19] |
| A. cyclinus (Shen, 1932) | Bivalvia | Barbatia virescens (Reeve, 1844), Cyclina sinensis (Gmelin, 1791), Meretrix meretrix (Linnaeus, 1758) | IWP | [74] |
| A. exiguus (Bürger, 1895) | ? | ? | IWP | [19] |
| A. guinotae Campos, 2001 | Bivalvia | Barbatia sp. | IWP | [115] |
| A. latifrons (Bürger, 1895) | ? | ? | IWP | [19] |
| A. latus (Bürger, 1895) 3 | Bivalvia | Atrina vexillum (Born, 1778), Pinna sp. | IWP | [19] |
| A. modiolicola (Bürger, 1895) | Bivalvia | Mactra violacea (Gmelin, 1791), Marcia opima (Gmelin, 1791), Modiolus philippinarum (Hanley, 1843) | IWP | [6,19] |
| A. nudifrons (Bürger, 1895) | ? | ? | IWP | [19] |
| A. obesus (Dana, 1852) | Bivalvia | Arca sp., Cytherea sp., Gafrarium spp., Sunetta subquadrata (Sowerby, 1851) | IWP | [6,49] |
| A. ocularius Komai et al., 2020 | Bivalvia | Andara spp. | IWP | [49] |
| A. palaensis (Bürger, 1895) | Bivalvia | Anadara antiquata (Linnaeus, 1758), Arca sp., Barbatia foliata (Forsskål in Niebuhr, 1775), Mactra grandis Gmelin, 1791, Placuna ephippium (Philipsson, 1788), Tegillarca granosa (Linnaeus, 1758) | IWP | [6] |
| A. pernicola (Bürger, 1895) | Bivalvia | Perna sp. | IWP | [19] |
| A. placunae (Hornell & Southwell, 1909) | Bivalvia | Placuna placenta (Linnaeus, 1758) | IWP | [6] |
| A. placunicola Ng, 2018 | Bivalvia | Placuna ephippium (Philipsson, 1788) | IWP | [157] |
| A. pollus Ahyong & Ng, 2020 | Bivalvia | Malleus albus (Lamarck, 1819) | IWP | [32] |
| A. purpureus (Alcock, 1900) | Bivalvia | Ostrea sp., Protapes gallus (Gmelin, 1791) | IWP | [158] |
| A. rayi Ahyong & Ng, 2007 | Bivalvia | ? | IWP | [19] |
| A. rhombifer (Bürger, 1895) | Bivalvia | Tucetona auriflua (Reeve, 1843) | IWP | [19] |
| A. ridgewayi (Southwell, 1911) 3 | Bivalvia | Pinna atropurpurea (Sowerby, 1825) | IWP | [19] |
| A. rotundatus (Bürger, 1895) | Bivalvia | Circe sp. | IWP | [19] |
| A. shahi Trivedi et al., 2018 | Bivalvia | Magallana bilineata (Röding, 1798) | IWP | [122] |
| A. similis (Bürger, 1895) | Bivalvia | Ostrea sp., Placuna placenta (Linnaeus, 1758) | IWP | [6] |
| A. sinensis (Shen, 1932) | Bivalvia | Alectryonella plicatula (Gmelin, 1791), Chlamys nipponensis (?), Magallana angulata (Lamarck, 1819), Magallana gigas (Thunberg, 1793), Meretrix lusoria (Röding, 1798), Modiolus auriculatus (Krauss, 1848), Mytilus unguiculatus Valenciennes, 1858, Mytilus edulis Linnaeus, 1758, Ruditapes philippinarum (Adams & Reeve, 1850), Venerupis aspera (Quoy & Gaimard, 1835) | IWP | [6,159] |
| A. spinidactylus (Gordon, 1936) | Bivalvia | Modiolus philippinarum (Hanley, 1843), Modiolus sp. | IWP | [6] |
| A. tivalae (Gordon, 1936) | Bivalvia | Tivela stefaninii (Nardini, 1933) | IWP | [6] |
| A. vicajii (Chhapgar, 1957) | Bivalvia | Marcia recens (Holten, 1802), Mercenaria sp., Meretrix casta (Gmelin, 1791), Perna viridis (Linnaeus, 1758) | IWP | [123,155] |
| A. winckworthi (Gordon, 1936) | Bivalvia | Protapes gallus (Gmelin, 1791) | IWP | [6] |
| Austinotheres | ||||
| A. angelicus (Lockington, 1877) | Bivalvia | Modiolus capax (Conrad, 1837), Ostrea angelica Rochebrune, 1895, Saccostrea palmula (Carpenter, 1857) | EP | [6,40] |
| Austrotheres | ||||
| A. holothuriensis (Baker, 1907) | Holothuria, Ascidia | Herdmania grandis (Heller, 1878), ‘holothurians’ | IWP | [12] |
| A. pregenzeri Ahyong, 2018 | Ascidia | Herdmania grandis (Heller, 1878) | IWP | [12] |
| Bonita | ||||
| B. mexicana Campos, 2009 | Bivalvia | Pseudochama exogyra (Conrad, 1837) | EP | [61] |
| Buergeres | ||||
| B. choprai Ahyong & Ng, 2020 | Holothuria | Actinopyga echinites (Jaeger, 1833) | IWP | [32] |
| B. deccanensis (Chopra, 1931) | Holothuria | Holothuria scabra Jaeger, 1833 | IWP | [99] |
| B. holothuriae (Semper, 1880) | Holothuria | Stichopus horrens Selenka, 1867 | IWP | [70] |
| B. ortmanni (Bürger, 1895) | Holothuria | Holothuria fursocinerea Jaeger, 1833 | IWP | [70] |
| Calyptraeotheres | ||||
| C. camposi Ayón-Parente & Hendrickx, 2014 | Gastropoda | Crepidula striolata Menke, 1851 | EP | [128] |
| C. garthi (Fenucci, 1975) | Gastropoda | Bostrycapulus odites Collin, 2005, Crepidula argentina Simone, Pastorino & Penchaszadeh, 2000, Crepidula cachimilla Cledón, Simone & Penchaszadeh, 2004, Crepidula plana Say, 1822, Crepidula protea (d’Orbigny, 1841), Crepidula unguiformis Lamarck, 1822, Crepidula sp., Trochita pileus (Lamarck, 1822) | ATL | [125,160] |
| C. granti (Glassell, 1933) | Gastropoda | Bostrycapulus odites Collin, 2005, Crepidula cachimilla Cledón, Simone & Penchaszadeh, 2004, Crucibulum spinosum (Sowerby, 1824), Crepidula striolata Menke, 1851, Lottia mesoleuca (Menke, 1851) | EP | [41,89] |
| C. hernandezi Hernández-Ávila & Campos, 2006 | Gastropoda | Crucibulum auricula (Gmelin, 1791) | ATL | [126] |
| C. pepeluisi Campos & Hernández-Ávila, 2010 | ? | ? | EP | [127] |
| C. politus (Smith, 1870) | Gastropoda | Calyptraea sp., Crepipatella dilatata (Lamarck, 1822) | EP | [125] |
| Clypeasterophilus | ||||
| C. juvenilis (Bouvier, 1917) | Echinoidea | Clypeaster subdepressus (Gray, 1825), Meoma ventricosa (Lamarck, 1816) | ATL | [161] |
| C. rugatus (Bouvier, 1917) | Echinoidea | Clypeaster rosaceus (Linnaeus, 1758), Encope michelini Agassiz, 1841 | ATL | [161] |
| C. stebbingi (Rathbun, 1918) | Echinoidea | Clypeaster subdepressus (Gray, 1825) | ATL | [161] |
| C. ususfructus (Griffith, 1987) | Echinoidea | Clypeaster europacificus Clark, 1914, Clypeaster speciosus Verrill, 1870 | EP | [161] |
| Discorsotheres | ||||
| D. camposi Ahyong, 2018 | Bivalvia | Spondylus spp. | IWP | [12] |
| D. spondylia (Nobili, 1905) | Bivalvia | Spondylus exilis cf. (Sowerby, 1895) | IWP | [12] |
| D. subglobosus (Baker, 1907) | Bivalvia | Equichlamys bifrons (Lamarck, 1819), Modiolus areolatus (Gould, 1850), Pecten fumatus Reeve, 1852, Spondylus tenellus Reeve, 1856 | IWP | [12] |
| D. subquadrata (T. Sakai, 1939) | Bivalvia | Magallana nippona (Reki, 1934), Mytilus edulis Linnaeus, 1758, Spondylus squamosus Schreibers, 1793 | IWP | [12] |
| Dissodactylus4 | ||||
| D. crinitichelis Moreira, 1901 | Echinoidea | Clypeaster rosaceus (Linnaeus, 1758), Clypeaster subdepressus (Gray, 1825), Encope emarginata (Leske, 1778), Encope michelini Agassiz, 1841, Leodia sexiesperforata (Leske, 1778), Mellita quinquiesperforata (Leske, 1778), Mellita sp., Meoma ventricosa (Lamarck, 1816) | ATL | [161] |
| D. glasselli Rioja, 1944 | Echinoidea | Encope micropora californica Verrill, 1870, Lanthonia grantii (Mortensen, 1948), Lanthonia longifissa (Michelin, 1858), Mellita kanakoffi Durham, 1961 | EP | [161] |
| D. latus H. Griffith, 1987 | Echinoidea | Clypeaster subdepressus (Gray, 1825), Encope michelini Agassiz, 1841, Leodia sexiesperforata (Leske, 1778) | ATL | [161] |
| D. lockingtoni Glassell, 1935 | Echinoidea | Encope grandis Agassiz, 1841, Encope micropora californica Verrill, 1870, Leodia sexiesperforata (Leske, 1778), Encope spp., Lanthonia grantii (Mortensen, 1948), Lanthonia longifissa (Michelin, 1858), Mellita kanakoffi Durham, 1961 | EP | [161] |
| D. mellitae (Rathbun, 1900) | Echinoidea | Clypeaster subdepressus (Gray, 1825), Encope michelini Agassiz, 1841, Leodia sexiesperforata (Leske, 1778), Mellita isometra Harold & Telford, 1990, Mellita quinquiesperforata (Leske, 1778) | ATL | [161] |
| D. nitidus Smith, 1870 | Echinoidea | Encope grandis Agassiz, 1841, Encope micropora californica Verrill, 1870, Encope spp., Lanthonia longifissa (Michelin, 1858) | EP | [161] |
| D. primitivus Bouvier, 1917 | Echinoidea | Meoma ventricosa (Lamarck, 1816), Plagiobrissus grandis (Gmelin, 1791) | ATL | [161] |
| D. schmitti H. Griffith, 1987 | ? | ? | EP | [93] |
| D. xantusi Glassell, 1936 | Echinoidea | Clypeaster subdepressus (Gray, 1825), Encope grandis Agassiz, 1841, Encope micropora californica Verrill, 1870, Encope michellini Agassiz, 1841, Encope spp., Leodia sexiesperforata (Leske, 1778), Mellitella stokesii (Agassiz, 1841), Lanthonia longifissa (Michelin, 1858) | EP | [161] |
| Durckheimia | ||||
| D. caeca Bürger, 1895 | Bivalvia | Chama pacifica Broderip, 1835, Lima lima (Linnaeus, 1758), Lima vulgaris (Link, 1807) | IWP | [27] |
| D. carnipes De Man, 1889 | ? | ? | IWP | [27] |
| D. lochi Ahyong & Brown, 2003 | Bivalvia | Ctenoides ales, Lima vulgaris (Link, 1807) | IWP | [27] |
| Enigmatheres | ||||
| E. canfieldi (Rathbun, 1918) | Gastropoda | Megathura crenulata (Sowerby, 1825) | EP | [61] |
| Ernestotheres | ||||
| E. conicola Manning, 1993 | Gastropoda | Conus sp. | ATL | [7] |
| Fabia5 | ||||
| F. byssomiae (Say, 1818) | Bivalvia | Hiatella arctica (Linnaeus, 1767), Anadara notabilis (Röding, 1798) | ATL | [16] |
| F. carvachoi Campos, 1996 | Bivalvia | Semele flavescens (Gould, 1851) | EP | [16] |
| F. concharum (Rathbun, 1894) | Bivalvia | Cryptomya californica (Conrad, 1837), Donax gouldii Dall, 1921, Leukoma staminea (Conrad, 1837), Modiolus capax (Conrad, 1837), Modiolus modiolus (Linnaeus, 1758), Mya Arenaria Linnaeus, 1758, Parapholas californica (Conrad, 1837), Pholadidea loscombiana Turton, 1819, Tapes sp., Tivela stultorum (Mawe, 1823) | EP | [16] |
| F. emiliai (Melo, 1971) | Bivalvia | Anadara brasiliana (Lamarck, 1819), Glycymeris longior (Sowerby, 1833), Glycymeris sp. | ATL | [116] |
| F. felderi Gore, 1986 | ? | ? | ATL | [116] |
| F. hemphilli (Rathbun, 1918) | ? | ? | ATL | [116] |
| F. malaguena (Garth, 1948) | ? | ? | EP | [162] |
| F. subquadrata Dana, 1851 | Bivalvia | Cyclocardia ventricosa (Gould, 1850), Leukoma staminea (Conrad, 1837), Modiolus capax (Conrad, 1837), Modiolus modiolus (Linnaeus, 1758), Mya arenaria Linnaeus, 1758, Mytilus californianus Conrad, 1837, Mytilus edulis Linnaeus, 1758, Saxidomus gigantea (Deshayes, 1839), Tresus capax (Gould, 1850), Tivela stultorum (Mawe, 1823), Tresus nuttallii (Conrad, 1837) | EP | [30] |
| F. tellinae Cobb, 1973 | Bivalvia | Laciolina magna (Spengler, 1798) | ATL | [16] |
| Gemmotheres | ||||
| G. chamae Roberts, 1975 | Bivalvia | Chama spp. | ATL | [85] |
| Holotheres | ||||
| H. danielae Ahyong, 2010 | Holothuria | Acaudina molpadioides (Semper, 1867) | IWP | [106] |
| H. flavus (Nauck, 1880) | Holothuria | ‘unidentified holothurian’ | IWP | [70] |
| H. halingi (Hamel et al., 1999) | Holothuria | Holothuria scabra Jaeger, 1833 | IWP | [120] |
| H. semperi (Bürger, 1895) | Holothuria | Holothuria fursocinerea Jaeger, 1833, Holothuria scabra Jaeger, 1833 | IWP | [70] |
| H. setnai (Chopra, 1931) | Holothuria | Actinopyga mauritiana (Quoy & Gaimard, 1834) | IWP | [6] |
| Holothuriophilus6 | ||||
| H. pacificus (Poeppig, 1836) | Holothuria | Athyonidium chilensis (Semper, 1868) | EP | [6] |
| H. trapeziformis Nauck, 1880 | Holothuria | Holothuria inornata (Semper, 1868) | EP | [41] |
| Hospitotheres | ||||
| H. powelli2 Manning, 1993 | Decapod burrows | Balsscallichirus balssi (Monod, 1933), Leptalpheus sp. nov. (?) | ATL | [7] |
| Juxtafabia | ||||
| J. muliniarum (Rathbun, 1918) | Bivalvia | Chione californiensis (Broderip, 1835), Chionista fructifraga (Sowerby, 1853), Chione tumens Verrill, 1870, Leukoma grata (Say, 1831), Tagelus affinis (Adams, 1852), Polymesoda inflata (Philippi, 1851) | EP | [163] |
| Latatheres | ||||
| L. affinis (H. Milne Edwards, 1853) | ? | ? | IWP | [12] |
| L. tomentipes (Takeda & Konichi, 1994) | Bivalvia | ‘unidentified bivalve mollusc’ | IWP | [12] |
| Limotheres | ||||
| L. nasatus Holthuis, 1975 | Bivalvia | Ctenoides mitis (Lamarck, 1807) | ATL | [55] |
| Mesotheres | ||||
| M. barbatus (Desbonne, 1867) | Gastropoda | Cittarium pica (Linnaeus, 1758) | ATL | [6] |
| M. serrei (Rathbun, 1909) | Gastropoda | Strombus sp. | ATL | [6] |
| M. strombi (Rathbun, 1905) | Gastropoda | Pleuroploca sp., Strombus alatus Gmelin, 1791, Strombus pugilis Linnaeus, 1758, Strombus sp., Triplofusus giganteus (Kiener, 1840) | ATL | [6,71] |
| M. unguifalcula (Glassel, 1936) 7 | Gastropoda | Strombus sp., Turbo sp. | EP | [164] |
| Nannotheres | ||||
| N. moorei Manning & Felder, 1996 | Bivalvia | Malleus candeanus (d’Orbigny, 1853) | ATL | [31] |
| Nepinnotheres5 | ||||
| N. affinis (Bürger, 1895) | Bivalvia | Chlamys hastata (Sowerby, 1842), Ostrea sp., Pinna sp. | IWP | [6] |
| N. africanus Manning, 1993 | ? | ? | ATL | [7] |
| N. androgynus Manning, 1993 | Bivalvia | Panopea glycimeris (Born, 1778) | ATL | [7] |
| N. atrinicola (Page, 1983) | Bivalvia | Atrina zelandica (Gray, 1835), Austrovenus stutchburyi (Wood, 1828), Modiolus areolatus (Gould, 1850) | IWP | [165] |
| N. cardii (Bürger, 1895) | Bivalvia | Fragum unedo (Linnaeus, 1758), Mactra chinensis Philippi, 1846, Mytilus unguiculatus Valenciennes, 1858, Meretrix meretrix (Linnaeus, 1758), Ostrea denselamellosa Lischke, 1869, Pinna bicolor Gmelin, 1791, Spisula sachalinensis (Schrenck, 1862) | IWP | [6] |
| N. edwardsi (De Man, 1887) | Bivalvia | Atrina vexillum (Born, 1778), Ostrea sp., Pinna sp. | IWP | [6] |
| N. fulvia Ahyong & Ng, 2020 | Bivalvia | Fulvia australis (Sowerby, 1834) | IWP | [32] |
| N. glaberrimus (Bürger, 1895) | Bivalvia | Arca sp., Ctenoides suavis Masahito, Kuroda & Habe in Kuroda & al., 1971, Geloina coaxans (Gmelin, 1791) | IWP | [6] |
| N. latipes (Jacquinot, 1846) | Bivales | Modiolus sp., Pinna sp., Saccostrea scyphophilla (Peron & Lesueur, 1807) | IWP | [166] |
| N. margaritiferae (Laurie, 1906) | Bivalvia | Mytilus sp., Pinctada imbricata (Röding, 1798) | IWP | [6] |
| N. novaezelandiae (Filhol, 1885) | Bivalvia | Aulacomya atra (Molina, 1782), Austrovenus stutchburyi (Wood, 1828), Magallana gigas (Thunberg, 1793), Mytilus galloprovincialis Lamarck, 1819, Paphies ventricosa (Gray, 1843), Perna canaliculus (Gmelin, 1791) | IWP | [165] |
| N. pectinicola (Bürger, 1895) | Bivalvia | Decatopecten radula (Linnaeus, 1758) | IWP | [6] |
| N. pinnotheres (Linnaeus, 1758) | Bivalvia, Ascidia | Atrina pectinata (Linnaeus, 1767), Pinna nobilis Linnaeus, 1758; Ascidia mentula Müller, 1776, Halocynthia papillosa (Linnaeus, 1767), Microcosmos spp. | ATL | [15,152] |
| N. rathbunae (Schmitt, 1973) | Bivalvia | Donax sp. | IWP | [6] |
| N. rouxi (H. Milne Edwards, 1853) | ? | ? | IWP | [6] |
| N. sanqueri Manning, 1993 | ? | ? | ATL | [7] |
| N. tellinae (Manning & Holthuis, 1981) | Bivalvia | Austromacoma nymphalis (Lamarck, 1818) | ATL | [7] |
| N. villosulus (Guérin, 1832) | Bivalvia | Atrina chinensis (Deshayes, 1841), Pinctada margaritifera (Linnaeus, 1758) | IWP | [6] |
| Opisthopus | ||||
| O. transversus Rathbun, 1894 8 | Polyplacophora, Gastropoda, Bivalvia, Polychaeta, Holothuria | Cryptochiton stelleri (Middendorff, 1847); Aplysia vaccaria Winkler, 1955, Bulla gouldiana Pilsbry, 1895, Megastraea undosa (Wood, 1828), Megathura crenulata (Sowerby, 1825), Navanax inermis (Cooper, 1862), Neverita lewisii (Gould, 1847); Crassadoma gigantea (Gray, 1825), Dinocardium robustum (Lightfoot, 1768), Megapitaria squalida (Sowerby, 1835), Modiolus sp., Mytilus edulis Linnaeus, 1758, Nuttallia nuttalli (Conrad, 1837), Pholas sp., Platyodon sp., Tivela stultorum (Mawe, 1823), Tresus nuttallii (Conrad, 1837), Zirfaea pilsbryi Lowe, 1931, Zirfaea sp.; Arenicola sp., Chaetopterus variopedatus (Renier, 1804); Apostichopus californicus (Stimpson, 1857), Apostichopus parvimensis (Clark, 1913), Caudina sp. | EP | [6,30] |
| Orthotheres | ||||
| O. bayou Ng & Ho, 2016 | Gastropoda | Haliotis asinine Linnaeus, 1758 | IWP | [112] |
| O. haliotidis Geiger & Martin, 1999 | Gastropoda | Haliotis asinina Linnaeus, 1758 | IWP | [112] |
| O. turboe T. Sakai, 1969 | Gastropoda | Turbo argyrostomus Linnaeus, 1758 | IWP | [112] |
| Ostracotheres | ||||
| O. cynthiae Nobili, 1906 | Ascidia | Herdmania momus (Savigny, 1816), Herdmania sp. | IWP | [12] |
| O. tridacnae (Rüppel, 1830) | Bivalvia | Tridacna maxima (Röding, 1798), Tridacna squamosina Sturany, 1899 | IWP | [12] |
| Pinnaxodes6 | ||||
| P. bipunctatus (Nicolet, 1849) | Echinoidea | ‘probably sea-urchins’ | EP | [167] |
| P. chilensis (H. Milne Edwards, 1837) | Echinoidea | Caenocentrotus gibbosus (Agassiz in Agassiz & Desor, 1846), Loxechinus albus (Molina, 1782), Tetrapygus niger (Molina, 1782) | EP | [167] |
| P. floridensis H.W. Wells & M.J. Wells, 1961 | Holothuria | Holothuria princeps Selenka, 1867 | ATL | [6] |
| P. gigas Green, 1992 | Bivalvia | Panopea generosa Gould, 1850, Panopea globose Dall, 1898, Pinna rugosa Sowerby, 1835 | EP | [30] |
| P. major Ortmann, 1894 | Bivalvia, Holothuria | Atrina pectinata (Linnaeus, 1767), Barnea sp., Crenomytilus grayanus (Dunker, 1853), Gregariella difficilis (Deshayes, 1863), Mactra antiquata Spengler, 1802, Meretrix lamarckii Deshayes, 1853, Mytilus sp., Ruditapes philippinarum (Adams & Reeve, 1850); Holothuria hilla Lesson, 1830, Stichopus gyrifer Selenka, 1867 | IWP | [6,87,168] |
| P. mutuensis T. Sakai, 1939 | Bivalvia | Crenomytilus grayanus (Dunker, 1853), Gregariella difficilis (Deshayes, 1863), Modiolus modiolus (Linnaeus, 1758), Mya arenenaria Linnaeus, 1758, Mytilus edulis Linnaeus, 1758, Mytilus galloprovincialis Lamarck, 1819, Mytilus unguiculatus Valenciennes, 1858 | IWP | [70,168] |
| P. tomensosus Ortmann, 1894 | Bivalvia | Anomalocardia flexuosa (Linnaeus, 1767), Cyrtopleura costata (Linnaeus, 1758) | ATL | [169] |
| Pinnotheres5 | ||||
| P. bicristatus García Raso & Cuesta, 2019 | Bivalvia | Anomia ephippium Linnaeus, 1758, Ostrea edulis Linnaeus, 1758 | ATL | [33,37] |
| P. bidentatus T. Sakai, 1939 9 | ‘Free living’ | ? | IWP | [110] |
| P. corbiculae T. Sakai, 1939 10 | Bivalvia | Corbicula japonica Prime, 1864 | IWP | [6] |
| P. coutierei Nobili, 1905 | ? | ? | IWP | [6] |
| P. dilatatus Shen, 1932 | Bivalvia | Venerupis aspera (Quoy & Gaimard, 1835) | IWP | [6] |
| P. excussus Tai et al., 1980 | Bivalvia | Gafrarium sp. | IWP | [74] |
| P. globosus Hombron & Jacquinot, 1846 3 | Bivalvia | Meretrix sp., Pinna sp., Sunetta subquadrata (Sowerby, 1851) | IWP | [6] |
| P. gordonae Shen, 1932 11 | Bivalvia | Anomia cytaeum Gray, 1850, Fulvia undatopicta (Pilsbry, 1904), Mytilus unguiculatus Valenciennes, 1858, Ruditapes philippinarum (Adams & Reeve, 1850), Venerupis aspera (Quoy & Gaimard, 1835), Venerupis philippinarum (Adams & Reeve, 1850) | IWP | [6] |
| P. guerini H. Milne Edwards, 1853 | Bivalvia | ‘oysters’ | ATL | [6] |
| P. haiyangensis Shen, 1932 | Bivalvia | Laternula gracilis (Reeve, 1860) | IWP | [6] |
| P. hickmani (Guiler, 1950) | Bivalvia | Eumarcia fumigata (Sowerby, 1853), Modiolus sp., Mytilus sp., Neotrigonia margaritacea (Lamarck, 1804), Plebidonax deltoides (Lamarck, 1818) | IWP | [53] |
| P. hirtimanus H. Milne Edwards, 1853 | ? | ? | ATL | [6] |
| P. kamensis Rathbun, 1909 | ? | ? | IWP | [6] |
| P. kutensis Rathbun, 1909 | ? | ? | IWP | [6] |
| P. lanensis Rathbun, 1909 | ? | ? | IWP | [6] |
| P. laquei T. Sakai, 1961 12 | Brachiopoda | Laqueus rubellus (Sowerby, 1846), ‘other brachiopods’ | IWP | [170] |
| P. lithodomi Smith, 1870 | Bivalvia | Leiosolenus attenuates (Deshayes, 1836), Lithophaga aristata (Dillwyn, 1817) | EP | [6] |
| P. luminatus Tai et al., 1980 | Bivalvia | Asaphis violascens (Forsskål in Niebuhr, 1775) | IWP | [74] |
| P. lutescens Nobili, 1905 | ? | ? | IWP | [6] |
| P. mactricola Alcock, 1900 | Bivalvia | Mactra violacea (Gmelin, 1791) | IWP | [6] |
| P. maindroni Nobili, 1905 | ? | ? | IWP | [6] |
| P. nigrans Rathbun, 1909 | ? | ? | IWP | [6] |
| P. obscuridentata Tai & Song, 1986 | ? | ? | IWP | [171] |
| P. obscurus Stimpson, 1858 | ? | ? | IWP | [6] |
| P. onychodactylus Tesch, 1918 | ? | ? | IWP | [6] |
| P. paralatissimus Tai & Song, 1986 | ? | ? | IWP | [171] |
| P. parvulus Stimpson, 1858 | Bivalvia | Pecten albicans (Schröter, 1802), Pinna sp., Saxidomus purpurata (Sowerby, 1852), Sunetta subquadrata (Sowerby, 1851) | IWP | [6] |
| P. pectunculi Hesse, 1872 | Bivalvia | Clausinella fasciata (da Costa, 1778), Glycymeris glycymeris (Linnaeus, 1758), Venus casina Linnaeus, 1758, Venus verrucosa Linnaeus, 1758 | IWP | [15] |
| P. perezi Nobili, 1905 | Bivalvia | Pholas sp. | IWP | [6] |
| P. pholadis De Haan, 1835 | Bivalvia | Barnea manilensis (Philippi, 1847), Chlamys nipponensis (?), Meretrix lusoria (Röding, 1798), Mimachlamys sanguinea (Linnaeus, 1758), Mytilus edulis Linnaeus, 1758, Pecten albicans (Schröter, 1802), Ruditapes philippinarum (Adams & Reeve, 1850), Spisula sachalinensis (Schrenck, 1862) | IWP | [6] |
| P. pichilinquei Rathbun, 1923 | ? | ? | EP | [6] |
| P. pilulus Tai et al., 1980 | Bivalvia | Martesia sp. | IWP | [74] |
| P. pisum (Linnaeus, 1767) | Bivalvia | Acanthocardia echinata (Linnaeus, 1758), Cerastoderma edule (Linnaeus, 1758), Chamelea gallina (Linnaeus, 1758), Clausinella fasciata (da Costa, 1778), Donax vittatus (da Costa, 1778), Dosinia lupinus (Linnaeus, 1758), Ensis ensis (Linnaeus, 1758), Ensis magnus Schumacher, 1817, Gari fervensis (Gmelin, 1791), Modiolus modiolus (Linnaeus, 1758), Mytilus edulis Linnaeus, 1758, Mytilus galloprovincialis Lamarck, 1819, Nucula nitidosa Winckworth, 1930, Ostrea edulis Linnaeus, 1758, Spisula spp. | ATL | [15] |
| P. pugettensis Holmes, 1900 | Ascidia, Bivalvia | Halocynthia aurantium (Pallas, 1787), Halocynthia igaboja Oka, 1906, Ascidia paratropa (Huntsman, 1912); Mya arenaria Linnaeus, 1758 | EP | [6] |
| P. quadratus Rathbun, 1909 | Bivalvia | Arca sp. | IWP | [6] |
| P. sebastianensis (Rodrigues da Costa, 1970) | Bivalvia | Atrina rigida (Lightfoot, 1786) | ATL | [6] |
| P. serrignathus Shen, 1932 | ? | ? | IWP | [6] |
| P. shoemaker Rathbun, 1918 | ? | ? | ATL | [6] |
| P. siamensis Rathbun, 1909 | ? | ? | IWP | [6] |
| P. taichungae K. Sakai, 2000 9 | ‘Free living’ | ? | IWP | [6] |
| P. taylori Rathbun, 1918 | Ascidia | ‘transparent tunicates’ | EP | [6] |
| P. trichopus Tesch, 1918 | Bivalvia | Pinctada sp. | IWP | [6] |
| P. tsingtaoensis Shen, 1932 | Bivalvia | Hiatula acuta (Cai & Zhuang, 1985), Laternula peichiliensi (?), Mactra quadrangularis Reeve, 1854 | IWP | [26,74] |
| Raytheres | ||||
| R. clavapedatus (Glassell, 1935) | Bivalvia | Leiosolenus attenuates (Deshayes, 1836) | EP | [6] |
| Serenotheres | ||||
| S. besutensis (Serène, 1967) | Bivalvia | Lithophaga sp. | IWP | [27] |
| S. janus Ng & Meyer, 2016 | Bivalvia | Leiosolenus obesus (Philippi, 1847) | IWP | [52] |
| Sindheres | ||||
| S. karachiensis Kazmi & Manning, 2003 | Bivalvia | Gastrochaena sp. | IWP | [105] |
| Solenotheres | ||||
| S. prolixus Ng & Ngo, 2010 | Bivalvia | Solen corneus Lamarck, 1818 | IWP | [101] |
| Tacitotheres | ||||
| T. glaber (Bürger, 1895) | Bivalvia | Tapes conspersus (Gmelin, 1791), Tapes literatus (Linnaeus, 1758) | IWP | [6] |
| T. laevis (Bürger, 1895) | Bivalvia | Coralliophaga sp. | IWP | [6] |
| T. longipes (Bürger, 1895) | ? | ? | IWP | [6] |
| Trichobezoares | ||||
| T. pilumnoides (Nobili, 1906) 13 | Holothuria | Holothuria scabra Jaeger, 1833 | IWP | [29] |
| T. villosissimus (Doflein, 1904) | Holothuria | Actinopyga mauritiana (Quoy & Gaimard, 1834) | IWP | [29] |
| Tridacnatheres | ||||
| T. whitei (de Man, 1888) | Bivalvia | Pinna sp., Tridacna gigas Linnaeus, 1758, Tridacna squamosa Lamarck, 1819 | IWP | [27] |
| Tumidotheres | ||||
| T. carabiensis Palacios Theil & Felder, 2019 | Bivalvia | Atrina rigida (Lightfoot, 1786), Barbatia candida (Helbling, 1779), Isognomon alatus (Gmelin, 1791), Pinctada imbricata Röding, 1798, Pteria colymbus (Röding, 1798) | ATL | [98] |
| T. maculatus (Say, 1818) 14 | Bivalvia | Aequipecten tehuelchus (d’Orbigny, 1842), Anomia simplex d’Orbigny, 1853, Argopecten gibbus (Linnaeus, 1758), Argopecten irradians (Lamarck, 1819), Atrina rigida (Lightfoot, 1786), Atrina seminuda (Lamarck, 1819), Atrina serrata (Sowerby, 1825), Chama macerophylla Gmelin, 1791, Modiolus americanus (Leach, 1815), Modiolus modiolus (Linnaeus, 1758), Mya arenaria Linnaeus, 1758, Mytilus edulis Linnaeus, 1758, Mytilus platensis d’Orbigny, 1842, Ostrea puelchana d’Orbigny, 1842, Perna perna (Linnaeus, 1758), Placopecten magellanicus (Gmelin, 1791) | ATL | [98] |
| T. margarita (Verrill, 1869) | Bivalvia | Argopecten irradians concentricus (Say, 1822), Argopecten ventricosus (Sowerby, 1842), Barbatia reeveana (d’Orbigny, 1846), Crassadoma gigantea (Gray, 1825), Limaria pacifica (d’Orbigny, 1846), Pinctada mazatlanica (Hanley, 1856) | EP | [8] |
| T. orcutti (Rathbun, 1918) | ? | ? | EP | [6] |
| Tunicotheres | ||||
| T. moseri (Rathbun, 1918) | Ascidia | Molgula occidentalis Traustedt, 1883, Phallusia nigra Savigny, 1816, Polycarpa spongiabilis Traustedt, 1883 | ATL | [8] |
| Viridotheres | ||||
| V. asaphis Ahyong, 2020 | Bivalvia | Asaphis violascens (Forsskål in Niebuhr, 1775) | IWP | [172] |
| V. buergeri (Rathbun, 1909) | ? | ? | IWP | [6] |
| V. cygnus Ahyong, 2020 | Bivalvia | Mactra pura Reeve, 1854, | IWP | [166] |
| V. gracilis (Bürger, 1895) | Bivalvia | Marcia opima (Gmelin, 1791), Solen sp. | IWP | [6] |
| V. kupang Ahyong, 2018 | ? | ? | IWP | [173] |
| V. lilliyae (Manning, 1993) | ? | ? | ATL | [7] |
| V. marionae Manning, 1996 | Bivalvia | Europicardium caparti (Nicklès, 1955) | ATL | [103] |
| V. otto Ahyong & Ng, 2007 | ? | ? | IWP | [19] |
| V. sanguinolariae (Pillai, 1951) | Bivalvia | Hiatula diphos (Linnaeus, 1771) | IWP | [6] |
| V. takedai Ahyong et al., 2012 | Bivalvia | Nipponoclava gigantea (Sowerby, 1888) | IWP | [174] |
| V. viridis (Manning, 1993) | ? | ? | ATL | [7] |
| Visayeres | ||||
| V. acron Ahyong & Ng, 2007 | Bivalvia | Lithophaga sp. | IWP | [54] |
| Waldotheres | ||||
| W. mccainae (Schmitt, 1973) | Bivalvia | Donax rugosus Linnaeus, 1758 | ATL | [7] |
| Xanthasia | ||||
| X. murigera White, 1846 | Bivalvia | Hippopus sp., Tridacna crocea Lamarck, 1819, Tridacna gigas (Linnaeus, 1758), Tridacna maxima (Röding, 1798), Tridacna squamosa Lamarck, 1819 | IWP | [27] |
| Zaops | ||||
| Z. angelae Manning, 1993 | Bivalvia | Brachidontes modiolus (Linnaeus, 1767) | ATL | [98] |
| Z. geddesi (Miers, 1880) | Bivalvia | Crassostrea rhizophorae (Guilding, 1828) | ATL | [6] |
| Z. ostreum (Say, 1817) | Bivalvia | Anomia peruviana d’Orbigny, 1846, Crassostrea rhizophorae (Guilding, 1828), Crassostrea virginica (Gmelin, 1791), Mytilus edulis Linnaeus, 1758, Pecten spp. | ATL | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Gier, W.; Becker, C. A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations. Diversity 2020, 12, 431. https://doi.org/10.3390/d12110431
de Gier W, Becker C. A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations. Diversity. 2020; 12(11):431. https://doi.org/10.3390/d12110431
Chicago/Turabian Stylede Gier, Werner, and Carola Becker. 2020. "A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations" Diversity 12, no. 11: 431. https://doi.org/10.3390/d12110431
APA Stylede Gier, W., & Becker, C. (2020). A Review of the Ecomorphology of Pinnotherine Pea Crabs (Brachyura: Pinnotheridae), with an Updated List of Symbiont-Host Associations. Diversity, 12(11), 431. https://doi.org/10.3390/d12110431