Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats
Abstract
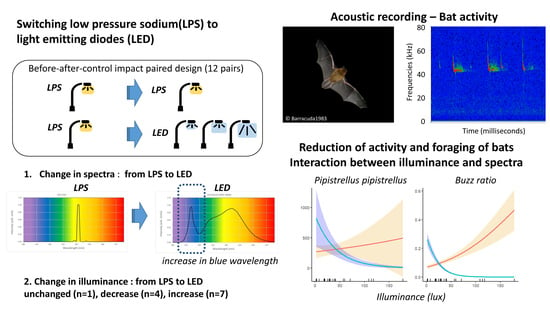
:1. Introduction
2. Material and Methods
2.1. Biological Model
2.2. Bat Activity
2.3. Experimental Set-up and Variation in Power and Illuminance of Lamps at Experimental Sites
2.4. Statistical Analyses
2.4.1. Preliminary Analysis 1: Analysis without Taking Account Changes in Light Intensity (Power and Illuminance)
2.4.2. Preliminary Analysis 2: Evaluation of the Influence of Intensity (Power and Illuminance) on Bats Activity
2.4.3. Disentangling the Relative Effects of Light Spectrum and Intensity (i.e., Power and Illuminance) on Bat Activity Following the Switch from LPS to LED Lamps
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hölker, F.; Moss, T.; Griefahn, B.; Kloas, W. The Dark Side of Light: A Transdisciplinary Research Agenda for Light Pollution Policy. Ecol. Soc. 2010, 15, 13. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal R. 2007, 43, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; p. 480. [Google Scholar]
- Azam, C.; Le Viol, I.; Julien, J.F.; Bas, Y.; Kerbiriou, C. Disentangling the relative effect of light pollution, im;pervious surfaces and intensive agriculture on bat activity with a national-scale monitoring program. Landsc. Ecol. 2016, 31, 2471–2483. [Google Scholar] [CrossRef] [Green Version]
- Laforge, A.; Pauwels, J.; Faure, B.; Bas, Y.; Kerbiriou, C.; Fonderflick, J.; Besnard, A. Reducing light pollution improves connectivity for bats in urban landscapes. Landsc. Ecol. 2019, 34, 793–809. [Google Scholar] [CrossRef]
- Stone, E.L.; Jones, G.; Harris, S. Street Lighting Disturbs Commuting Bats. Curr. Biol. 2009, 19, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.L.; Jones, G.; Harris, S. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Chang. Biol. 2012, 18, 2458–2465. [Google Scholar] [CrossRef]
- Davies, T.W.; Coleman, M.; Griffith, K.M.; Jenkins, S.R. Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 2015, 11, 20150080. [Google Scholar] [CrossRef]
- Minnaar, C.; Boyles, J.G.; Minnaar, I.A.; Sole, C.L.; Mckechnie, A.E. Stacking the odds: Light pollution may shift the balance in an ancient predator–prey arms race. J. Appl. Ecol. 2015, 52, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Bennie, J.; Davies, T.W.; Cruse, D.; Inger, R.; Gaston, K.J. Cascading effects of artificial light at night: Resource-mediated control of herbivores in a grassland ecosystem. Philos. Trans. R. Soc. B 2015, 370, 20140131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. The European GreenLight Programme Efficient Lighting Project Implementation Catalogue 2005–2009. 2011. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC62317/reqno_jrc62317_lb-na-24689-en.pdf (accessed on 27 March 2020).
- Almeida, P.S.; Braga, H.A.C.; Dalla Costa, M.A.; Alonso, J.M. Offline Soft-Switched LED Driver Based on an Integrated Bridgeless Boost–Asymmetrical Half-Bridge Converte. IEEE Trans. Ind. Appl. 2015, 51, 761–769. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Hänel, A.; Hölker, F. Redefining efficiency for outdoor lighting. Energy Environ. Sci. 2014, 7, 1806–1809. [Google Scholar] [CrossRef]
- Update on the Status of LED Market. Publications Office of the European Union, 2014. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC92971/jrc92971%20online.pdf (accessed on 27 March 2020).
- Rowse, E.G.; Harris, S.; Jones, G. The switch from low-pressure sodium to light emitting diodes does not affect bat activity at street lights. PLoS ONE 2016, 11, e0150884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubé, M.; Roby, J.; Kocifaj, M. Evaluating Potential Spectral Impacts of Various Artificial Lights on Melatonin Suppression, Photosynthesis, and StarVisibility. PLoS ONE 2013, 8, e67798. [Google Scholar] [CrossRef] [Green Version]
- Dominoni, D.M.; Quetting, M.; Partecke, J. Long-Term Effects of Chronic Light Pollution on Seasonal Functions of European Blackbirds (Turdus merula). PLoS ONE 2013, 8, e85069. [Google Scholar] [CrossRef]
- Durrant, J.; Michaelides, E.B.; Rupasinghe, T.; Tull, D.; Green, M.P.; Jones, T.M. Constant illumination reduces circulating melatonin and impairs immune function in the cricket Teleogryllus commodus. PeerJ 2015, 3, e1075. [Google Scholar] [CrossRef] [Green Version]
- van Langevelde, F.; Ettema, J.A.; Donners, M.; WallisDeVries, M.F.; Groenendijk, D. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 2011, 144, 2274–2281. [Google Scholar] [CrossRef]
- Rydell, J. Exploitation of insects around streetlamps by bats in Sweden. Funct. Ecol. 1992, 6, 744–750. [Google Scholar] [CrossRef]
- Azam, C.; Kerbiriou, C.; Vernet, A.; Julien, J.F.; Bas, Y.; Plichard, L.; Maratrat, J.; Le Viol, I. Is part-night lighting an effective measure to limit the impacts of artificial lighting on bats? Glob. Chang. Biol. 2015, 21, 4333–4341. [Google Scholar] [CrossRef]
- Lacoeuilhe, A.; Machon, N.; Julien, J.-F.; Le Bocq, A.; Kerbiriou, C. The influence of low intensities of light pollution on bat communities in a semi-natural context. PLoS ONE 2014, 9, e103042. [Google Scholar] [CrossRef]
- Eisenbeis, G. Artificial night lighting and insects: Attraction of insects to streetlamps in a rural setting in Germany. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 281–304. [Google Scholar]
- Rydell, J. Bats and Their Insect Prey at Streetlights. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 43–60. [Google Scholar]
- Stone, E.; Zeale, M.R.K.; Newson, S.E.; Browne, W.J.; Harris, S.; Jones, G. Managing Conflict between Bats and Humans: The Response of Soprano Pipistrelles (Pipistrellus pygmaeus) to Exclusion from Roosts in Houses. PLoS ONE 2015, 10, e0131825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azam, C.; Le Viol, I.; Bas, Y.; Zissis, G.; Vernet, A.; Julien, J.F.; Kerbiriou, C. Evidence for distance and illuminance thresholds in the effects of artificial lighting on bat activity. Landsc. Urban Plan. 2018, 175, 123–135. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 322–342. [Google Scholar]
- Owens, A.C.S.; Lewis, S.M. The impact of artificial light at night on nocturnal insects: A review and synthesis. Ecol. Evol. 2018, 11337–11358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Rydell, J. Foraging strategy and predation risk as factors influencing emergence time in echolocating bats. Philos. Trans. R. Soc. B. 1994, 346, 445–455. [Google Scholar]
- Rydell, J.; Entwistle, A.; Racey, A. Timing of Foraging Flights of Three Species of Bats in Relation to Insect Activity and Predation Risk. Oikos 1996, 76, 243–252. [Google Scholar] [CrossRef]
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. A review of the global conservation status of bats. Oryx 2002, 36, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Arlettaz, R.; Godat, S.; Meyer, H. Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biol. Conserv. 2000, 93, 55–60. [Google Scholar] [CrossRef]
- Robinson, M.F.; Stebbings, R.E. Home range and habitat use by the serotine bat, Eptesicus serotinus, in England. J. Zool. 1997, 243, 117–136. [Google Scholar] [CrossRef]
- Safi, K.; König, B.; Kerth, G. Sex differences in population genetics, home range size and habitat use of the parti-colored bat (Vespertilio murinus, Linnaeus 1758) in Switzerland and their consequences for conservation. Biol. Conserv. 2007, 137, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Spoelstra, K.; van Grunsven, R.H.A.; Ramakers, J.J.C.; Ferguson, K.B.; Raap, T.; Donners, M.; Veenendaal, E.M.; Visser, M.E. Response of bats to light with different spectra: Light-shy and agile bat presence is affected by white and green, but not red light. Proc. R. Soc. Ser. B-Biol. Soc. 2017, 284, 1855. [Google Scholar] [CrossRef] [PubMed]
- Obrist, M.K.; Boesch, R.; Flückiger, P.F. Variability in echolocation call design of 26 Swiss bat species: Consequences, limits and options for automated field identification with a synergetic pattern recognition approach. Mammalia 2004, 68, 307–322. [Google Scholar] [CrossRef] [Green Version]
- Britton, A.R.; Jones, G. Echolocation behaviour and prey-capture success in foraging bats: Laboratory and field experiments on Myotis daubentonii. J. Exp. Biol. 1999, 202, 1793–1801. [Google Scholar] [PubMed]
- Hayes, J.P. Temporal Variation in Activity of Bats and the Design of Echolocation-Monitoring Studies. J. Mam. 1997, 78, 21. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, C.F.J. Influence of season, habitat, temperature, and invertebrate availability on nocturnal activity of the New Zealand long-tailed bat (Chalinolobus tuberculatus). N. Z. J. Zool. 2000, 27, 207–221. [Google Scholar] [CrossRef]
- Ciechanowski, M.; Zajac, T.; Bilas, A.; Dunajski, R. Spatiotemporal variation in activity of bat species differing in hunting tactics: Effects of weather, moonlight, food abundance, and structural clutter. Can. J. Zool. 2007, 85, 1249–1263. [Google Scholar] [CrossRef]
- Newson, S.E.; Evans, H.E.; Gillings, S. A novel citizen science approach for large-scale standardised monitoring of bat activity and distribution, evaluated in eastern England. Biol. Conserv. 2015, 191, 38–49. [Google Scholar] [CrossRef]
- Generalized Linear Mixed Models Using Template Model Builder. 2020. Available online: http://cran.uni-muenster.de/web/packages/glmmTMB/glmmTMB.pdf (accessed on 27 March 2020).
- Freckleton, R.P. On the misuse of residuals in ecology: Regression of residuals vs. multiple regression. J. Anim. Ecol. 2002, 71, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Kerbiriou, C.; Bas, Y.; Le Viol, I.; Lorrillière, R.; Mougnot, J.; Julien, J.F. Bat Pass Duration Measurement: An Indirect Measure of Distance of Detection. Diversity 2019, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Meth. Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Nelson, R.J. Artificial light at night as an environmental pollutant: An integrative approach across taxa, biological functions, and scientific disciplines. J. Exp. Zool. 2018, 329, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Polak, T.; Korine, C.; Yair, S.; Holderied, M.W. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zool. 2011, 285, 21–27. [Google Scholar]
- Boldogh, S.; Dobrosi, D.; Samu, P. The effects of the illumination of buildings on house-dwelling bats and its conservation consequences. Acta Chiropterol. 2007, 9, 527–534. [Google Scholar] [CrossRef]
- Stone, E.L.; Wakefield, A.; Harris, S.; Jones, G. The impacts of new street light technologies: Experimentally testing the effects on bats of changing from low-pressure sodium to white metal halide. Philos. Trans. R. Soc. B 2015, 370, 20140127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewanzik, D.; Voigt, C.C. Transition from conventional to light-emitting diode street lighting changes activity of urban bats. J. Appl. Ecol. 2017, 54, 264–271. [Google Scholar] [CrossRef]
- Perkin, E.K.; Hölker, F.; Tockner, K.; Richardson, J.S. Artificial light as a disturbance to light-naïve streams. Freshw. Biol. 2014, 59, 2235–2244. [Google Scholar] [CrossRef]
- Grubisic, M.; van Grunsven, R.; Kyba, C.; Manfrin, A.; Hölker, F. Insect declines and agroecosystems: Does light pollution matter? Ann. Appl. Biol. 2018, 173, 180–189. [Google Scholar] [CrossRef]
- Conrad, K.F.; Warren, M.S.; Fox, R.; Parsons, M.S.; Woiwod, I.P. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 2006, 132, 279–291. [Google Scholar] [CrossRef]
- Pauwels, J.; Le Viol, I.; Azam, C.; Valet, N.; Julien, J.F.; Bas, Y.; Lemarchand, C.; de Miguel, A.S.; Kerbiriou, C. Accounting for artificial light impact on bat activity for a biodiversity-friendly urban planning. Landsc. Urban Plan. 2019, 183, 12–25. [Google Scholar] [CrossRef]
- Hale, J.D.; Fairbrass, A.J.; Matthews, T.J.; Davies, G.; Sadler, J.P. The ecological impact of city lighting scenarios: Exploring gap crossing thresholds for urban bats. Glob. Chang. Biol. 2015, 21, 2467–2478. [Google Scholar] [CrossRef] [Green Version]
- Bennie, J.; Davies, T.W.; Inger, R.; Gaston, K.J. Mapping artificial lightscapes for ecological studies. Met. Ecol. Evol. 2014, 5, 534–540. [Google Scholar] [CrossRef]
- Sánchez de Miguel, A.; Aubé, M.; Zamorano, J.; Kocifaj, M.; Roby, J.; Tapia, C. Sky Quality Meter measurements in a colour-changing world. Mon. Not. R. Astron. Soc. 2017, 467, 2966–2979. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, T.W.; Bennie, J.; Hopkins, J. REVIEW: Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49, 1256–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Light Intensity | Mean (±SE) | Minimum | Maximum |
|---|---|---|---|
| Changes of power (watts) | −10.6 ± 3.8 (−40%) | −21 (−61%) | +16 (+18%) |
| Changes of illuminance (lux) | +17.7 ± 8.5 (+259%) | −21 (−51%) | +64 (+1800%) |
| Bat Activities | Illuminance | Power | |||
|---|---|---|---|---|---|
| Estimate ± SE | p−Value | Estimate ± SE | p−Value | ||
| Total bat activity | Intercept | 3.670 ± 0.917 | <0.001 | 3.713 ± 0.898 | <0.001 |
| LampLPS | −0.733 ± 0.393 | 0.062 | −0.988 ± 0.456 | 0.030 | |
| Lamp intensity | 0.001 ± 0.007 | 0.896 | 0.001 ± 0.010 | 0.945 | |
| LampLPS:Lamp intensity | 0.021 ± 0.007 | 0.003 | 0.022 ± 0.010 | 0.029 | |
| Pipistrellus pipistrellus | Intercept | 6.760 ± 0.586 | <0.001 | 6.584 ± 0.522 | <0.001 |
| LampLPS | −1.168 ± 0.566 | 0.039 | −0.732 ± 0.625 | 0.241 | |
| Lamp intensity | −0.024 ± 0.009 | 0.005 | −0.033 ± 0.012 | 0.004 | |
| LampLPS:Lamp intensity | 0.027 ± 0.010 | 0.008 | 0.030 ± 0.014 | 0.033 | |
| Pipistrellus pygmaeus | Intercept | 2.180 ± 0.935 | 0.020 | 2.578 ± 0.799 | 0.001 |
| LampLPS | −0.318 ± 0.922 | 0.730 | −1.248 ± 0.918 | 0.174 | |
| Lamp intensity | 0.027 ± 0.015 | 0.080 | 0.031 ± 0.016 | 0.052 | |
| LampLPS:Lamp intensity | −0.001 ± 0.017 | 0.968 | 0.003 ± 0.018 | 0.886 | |
| Nyctalus spp. | Intercept | 2.093 ± 0.786 | 0.008 | 2.335 ± 0.808 | 0.004 |
| LampLPS | −0.773 ± 0.547 | 0.158 | −1.578 ± 0.712 | 0.027 | |
| Lamp intensity | −0.001 ± 0.010 | 0.886 | −0.014 ± 0.017 | 0.403 | |
| LampLPS:Lamp intensity | 0.032 ± 0.009 | 0.001 | 0.051 ± 0.014 | <0.001 | |
| Feeding buzz ratio | Intercept | −0.931 ± 0.237 | <0.001 | −0.662 ± 0.262 | 0.012 |
| LampLPS | −1.681 ± 0.188 | <0.001 | −2.415 ± 0.294 | <0.001 | |
| Lamp intensity | −0.052 ± 0.004 | <0.001 | −0.132 ± 0.009 | <0.001 | |
| LampLPS:Lamp intensity | 0.066 ± 0.004 | <0.001 | 0.154 ± 0.010 | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerbiriou, C.; Barré, K.; Mariton, L.; Pauwels, J.; Zissis, G.; Robert, A.; Le Viol, I. Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity 2020, 12, 165. https://doi.org/10.3390/d12040165
Kerbiriou C, Barré K, Mariton L, Pauwels J, Zissis G, Robert A, Le Viol I. Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity. 2020; 12(4):165. https://doi.org/10.3390/d12040165
Chicago/Turabian StyleKerbiriou, Christian, Kévin Barré, Léa Mariton, Julie Pauwels, Georges Zissis, Alexandre Robert, and Isabelle Le Viol. 2020. "Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats" Diversity 12, no. 4: 165. https://doi.org/10.3390/d12040165
APA StyleKerbiriou, C., Barré, K., Mariton, L., Pauwels, J., Zissis, G., Robert, A., & Le Viol, I. (2020). Switching LPS to LED Streetlight May Dramatically Reduce Activity and Foraging of Bats. Diversity, 12(4), 165. https://doi.org/10.3390/d12040165