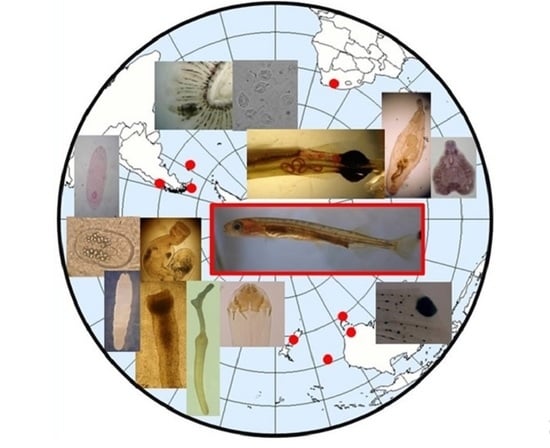
A Global Assessment of Parasite Diversity in Galaxiid Fishes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Predictors of Parasite Diversity
2.3. Statistical Analysis
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Base Variable | Country | Slope Estimate | SE | 95% Confidence Interval (Lower, Upper) |
| N studies | Argentina | 18.94 | 1.10 | 16.68, 21.19 |
| Australia | 6.18 | 0.51 | 5.13, 7.22 | |
| Chile | 9.08 | 0.66 | 7.71, 10.44 | |
| New Zealand | 6.23 | 0.42 | 5.37, 7.09 | |
| Latitudinal range size | Argentina | −31.44 | 31.80 | −96.93, 34.06 |
| Australia | 4.57 | 1.63 | 1.22, 7.93 | |
| Chile | 3.78 | 3.95 | −4.35, 11.92 | |
| New Zealand | 6.42 | 1.75 | 2.82, 10.02 | |
| Latitudinal Range Size | N Studies | Trend Estimate | SE | Asymptotic 95% Confidence Interval (Lower, Upper) |
| 1 | 0.0008 | 0.0002 | 0.0003, 0.001 | |
| 5 | 0.0006 | 0.0002 | 0.0002, 0.001 | |
| 10 | 0.0003 | 0.0003 | −0.0003, 0.0001 | |
| 15 | <0.0001 | 0.0005 | −0.001, 0.001 |
| Base Variable | Contrast | Estimate | SE | t Ratio | p |
|---|---|---|---|---|---|
| N studies | Argentina–Australia | 12.76 | 1.21 | 10.56 | <0.001 |
| Argentina–Chile | 9.86 | 1.28 | 7.70 | <0.001 | |
| Argentina–New Zealand | 12.70 | 1.17 | 10.83 | <0.001 | |
| Australia–Chile | −2.90 | 0.83 | −3.47 | <0.001 | |
| Australia–New Zealand | −0.05 | 0.66 | −0.08 | 1.000 | |
| Chile–New Zealand | 2.84 | 0.78 | 3.63 | 0.007 | |
| Latitudinal range size | Argentina–Australia | −36.01 | 31.84 | −1.13 | 0.674 |
| Argentina–Chile | −35.22 | 32.04 | −1.10 | 0.693 | |
| Argentina–New Zealand | −37.85 | 31.85 | −1.19 | 0.640 | |
| Australia–Chile | 0.79 | 4.27 | 0.19 | 0.998 | |
| Australia–New Zealand | −1.84 | 2.39 | −0.77 | 0.866 | |
| Chile–New Zealand | −2.63 | 4.32 | −0.61 | 0.928 |
| Contrast | Estimate | SE | z Ratio | p |
|---|---|---|---|---|
| Argentina–Australia | 1.88 | 0.48 | 3.93 | <0.001 |
| Argentina–Chile | 1.37 | 0.54 | 2.54 | 0.054 |
| Argentina–New Zealand | 1.96 | 0.48 | 4.10 | <0.001 |
| Australia–Chile | −0.51 | 0.59 | −0.86 | 0.826 |
| Australia–New-Zealand | 0.08 | 0.53 | 0.15 | 0.999 |
| Chile–New-Zealand | 0.59 | 0.59 | 0.99 | 0.753 |
References
- Gómez, A.; Nichols, E. Neglected wild life: Parasitic biodiversity as a conservation target. Int. J. Parasitol. Parasites Wildl. 2013, 2, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 20 September 2020).
- Colwell, R.K.; Dunn, R.R.; Harris, N.C. Coextinction and persistence of dependent species in a changing world. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 183–203. [Google Scholar] [CrossRef] [Green Version]
- Koh, L.P.; Dunn, R.R.; Sodhi, N.S.; Colwell, R.K.; Proctor, H.C.; Smith, V.S. Species coextinctions and the biodiversity crisis. Science 2004, 305, 1632–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, R.R.; Harris, N.C.; Colwell, R.K.; Koh, L.P.; Sodhi, N.S. The sixth mass coextinction: Are most endangered species parasites and mutualists? Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 3037–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, T.L.; Mora, C.; Rohde, K. Patterns of diversity and distribution of aquatic invertebrates and their parasites. In Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics; Morand, S., Krasnov, B., Littlewood, D., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 39–57. [Google Scholar]
- Carlson, C.J.; Dallas, T.A.; Alexander, L.W.; Phelan, A.; Phillips, A.J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 2020, 287, 815902. [Google Scholar] [CrossRef]
- Okamura, B.; Hartigan, A.; Naldoni, J. Extensive uncharted biodiversity: The parasite dimension. Integr. Comp. Biol. 2018, 58, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; O’Dwyer, K.; Nakagawa, S.; Poulin, R. Host diversity drives parasite diversity: Meta-analytical insights into patterns and causal mechanisms. Ecography 2014, 37, 689–697. [Google Scholar] [CrossRef]
- Poulin, R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef]
- Randhawa, H.S.; Poulin, R. Determinants of tapeworm species richness in elasmobranch fishes: Untangling environmental and phylogenetic influences. Ecography 2010, 33, 866–877. [Google Scholar] [CrossRef]
- Morand, S. (macro-) Evolutionary ecology of parasite diversity: From determinants of parasite species richness to host diversification. Int. J. Parasitol. Parasites Wildl. 2015, 4, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, T.; O’Dwyer, K.; Nakagawa, S.; Poulin, R. What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol. Rev. 2014, 89, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Guégan, J.-F.; Lambert, A.; Lévêque, C.; Combes, C.; Euzet, L. Can host body size explain the parasite species richness in tropical freshwater fishes? Oecologia 1992, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Price, P.W.; Clancy, K.M. Patterns in number of helminth parasite species in freshwater fishes. J. Parasitol. 1983, 449–454. [Google Scholar] [CrossRef]
- Walther, B.A.; Cotgreave, P.; Price, R.D.; Gregory, R.D.; Clayton, D.H. Sampling effort and parasite species richness. Parasitol. Today 1995, 11, 306–310. [Google Scholar] [CrossRef]
- Sarabeev, V.; Balbuena, J.A.; Morand, S. Testing the enemy release hypothesis: Abundance and distribution patterns of helminth communities in grey mullets (Teleostei: Mugilidae) reveal the success of invasive species. Int. J. Parasitol. 2017, 47, 687–696. [Google Scholar] [CrossRef]
- Poulin, R.; Presswell, B.; Jorge, F. The state of fish parasite discovery and taxonomy: A critical assessment and a look forward. Int. J. Parasitol. 2020, 50, 733–742. [Google Scholar] [CrossRef]
- Poulin, R.; Leung, T. Taxonomic resolution in parasite community studies: Are things getting worse? Parasitology 2010, 137, 1967–1973. [Google Scholar] [CrossRef]
- Allibone, R. Dealing with diversity dwarf galaxias style. Water Atmos. 2002, 10, 18–19. [Google Scholar]
- Eldon, A.E. White spots red faces. Freshw. Catch 1989, 40, 10. [Google Scholar]
- Blasco-Costa, I.; Cutmore, S.C.; Miller, T.L.; Nolan, M.J. Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Syst. Parasitol. 2016, 93, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Perkins, S.; Martinsen, E.; Falk, B. Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 2011, 138, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.A.; de León, G.P. Integrating molecular and morphological approaches for characterizing parasite cryptic species: Implications for parasitology. Parasitology 2011, 138, 1688–1709. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R. Crying wolf, crying foul, or crying shame: Alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Rev. Fish Biol. Fish. 2006, 16, 233–422. [Google Scholar] [CrossRef]
- Cussac, V.E.; Barrantes, M.E.; Boy, C.C.; Górski, K.; Habit, E.; Lattuca, M.E.; Rojo, J.H. New insights into the distribution, physiology and life histories of South American galaxiid fishes, and potential threats to this unique fauna. Diversity 2020, 12, 178. [Google Scholar] [CrossRef]
- Murillo, V.; Ruiz, V. El puye Galaxias globiceps Eigenmann 1927 (Osteichthyes: Galaxiidae): ¿Una especie en peligro de extinción? Gayana 2001, 66, 191–197. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P.; Burridge, C.P.; Craw, D. Geology shapes biogeography: Quaternary river-capture explains New Zealand’s biologically ‘composite’ Taieri River. Quat. Sci. Rev. 2015, 120, 47–56. [Google Scholar] [CrossRef]
- Craw, D.; Upton, P.; Burridge, C.P.; Wallis, G.P.; Waters, J.M. Rapid biological speciation driven by tectonic evolution in New Zealand. Nat. Geosci. 2016, 9, 140–144. [Google Scholar] [CrossRef]
- Meijer, C.G.; Warburton, H.J.; Harding, J.S.; McIntosh, A.R. Shifts in population size structure for a drying-tolerant fish in response to extreme drought. Austral Ecol. 2019, 44, 658–667. [Google Scholar] [CrossRef]
- Habit, E.; Piedra, P.; Ruzzante, D.E.; Walde, S.J.; Belk, M.C.; Cussac, V.E.; Gonzalez, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Díaz, G. Revealing the Effects of Loss of Longitudinal Connectivity on Freshwater Fish in Andean River Networks. Ph.D. Thesis, University of Concepción, Concepción, Chile, 2019. [Google Scholar]
- McDowall, R.M. Accumulating evidence for a dispersal biogeography of southern cool temperate freshwater fishes. J. Biogeogr. 2002, 29, 207–219. [Google Scholar] [CrossRef]
- Hine, M.; Jones, J.B.; Diggles, B.K. A Checklist of the Parasites of New Zealand Fishes Including Previously Unpublished Records; The National Institute of Water and Atmospheric Research: Wellington, New Zealand, 2000; p. 95. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 4 March 2020).
- Johnson, W.; Mace, J.; Turner, A. Fisheries Survey of Lake Christabel, West Coast Acclimatisation District, South Island; Fisheries Technical Report 144; Ministry of Agriculture and Fisheries: Wellington, New Zealand, 1976; p. 28.
- Tritt, E. Black Spot Disease in Freshwater Fishes of South-Western Australia: Identification of the Parasite, Host Range and Potential as a Bioindicator for Water Quality. Ph.D. Thesis, Murdoch University, Perth, Australia, 2018. [Google Scholar]
- Semenas, L. Estructura Comunitaria de Parásitos en Galaxias maculatus (Pisces, Galaxiidae) y Percichthys trucha (Pisces, Percichthydae) del lago Escondido (Río Negro, Argentina). Ph.D. Thesis, Universidad Nacional de Buenos Aires, Buenos Aires, Argentina, 1999. [Google Scholar]
- Trochine, C. Infestación por Acanthostomoides apophalliformis (Trematoda, Acanthostomidae) de la Fauna íctica del Sistema del lago Moreno. Licenciate Thesis, Universidad Nacional del Comahue, Bariloche, Argentina, 2000. [Google Scholar]
- Brunsdon, R.V. Studies on Nematode Parasites of New Zealand Fishes. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 1956. [Google Scholar]
- Hewitt, G.C.; Hine, P.M. Checklist of parasites of New Zealand fishes and of their hosts. N. Z. J. Mar. Freshw. Res. 1972, 6, 69–114. [Google Scholar] [CrossRef]
- Okamura, B. Hidden infections and changing environments. Integr. Comp. Biol. 2016, 56, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, P.; Franjola, R.; Cabezas, X.; Neira, A.; Covarrubias, C. Distribución de la infección por Camallanus corderoi: Nemata; spiruroidea; en distintos hospedadores autóctonos y sectores de la cuenca del río Valdivia, Chile. Bol. Chil. Parasitol. 1990, 45, 55–59. [Google Scholar] [PubMed]
- Flores, V.; Semenas, L. Infection patterns of Tylodelphys barilochensis and T. crubensis (Trematoda: Diplostomatidae) metacercariae in Galaxias maculatus (Osmeriformes: Galaxiidae) from two Patagonian lakes and observations on their geographical distribution in the southern Andean region, Argentina. J. Parasitol. 2002, 88, 1135–1139. [Google Scholar] [CrossRef]
- Paterson, R.A.; Townsend, C.R.; Poulin, R.; Tompkins, D.M. Introduced brown trout alter native acanthocephalan infections in native fish. J. Anim. Ecol. 2011, 80, 990–998. [Google Scholar] [CrossRef]
- Viozzi, G.; Semenas, L. Do environmental differences between lakes in Northwestern Argentinean Patagonia affect the infection of Philureter trigoniopsis (Monogenea) in Galaxias maculatus (Osmeriformes)? J. Parasitol. 2009, 95, 25–31. [Google Scholar] [CrossRef]
- McDowall, R.M. Making a living in Red Pond: A snapshot of the diet of a population of Aplochiton zebra (Teleostei: Galaxiidae) at the Falkland Islands. N. Z. J. Zool. 2005, 32, 23–27. [Google Scholar] [CrossRef]
- Macfarlane, W.V. Life cycle of Coitocaecum anaspidis Hickman, a New Zealand digenetic trematode. Parasitology 1939, 31, 172–184. [Google Scholar] [CrossRef]
- Holton, A.L. A redescription of Coitocaecum parvum Crowcroft, 1945 (Digenea: Allocreadiidae) from crustacean and fish hosts in Canterbury. N. Z. J. Zool. 1984, 11, 1–8. [Google Scholar] [CrossRef]
- Torres, P.; Franjola, R.; Cubillos, V.; Miranda, J.C.; Vera, R. Parasitismo en ecosistemas de agua dulce en Chile. 1. Presencia de metacercarias del género Stephanostomum (Digenea: Acanthocolpidae) en peces. J. Vet. Med. B 1988, 35, 169–177. [Google Scholar] [CrossRef]
- Torres, P.; Franjola, T.R.; Montefusco, A. Infección estacional por metacercarias de Diplostomum (Austrodiplostomum) mordax (Szidat y Nani, 1951) y Tylodelphys destructor Szidat y Nani, 1951 en el pejerrey chileno, Basilichthys australis Eigenmann, 1927 (Pisces: Atherinidae) en el lago Riñigue, Chile. Bol. Chil. Parasitol. 1996, 51, 15–19. [Google Scholar] [PubMed]
- Ostrowski de Núñez, M.; Semenas, L.; Brugni, N.; Viozzi, G.; Flores, V. Redescription of Acanthostomoides apophalliformis (Trematoda, Acanthostomidae) from Percichthys trucha. Acta Parasitol. 1999, 44, 222–228. [Google Scholar]
- Torres, P.; Franjola, R.; Pérez, J.; Auad, S.; Uherek, F.; Miranda, J.C.; Flores, L.; Riquelme, J.; Salazar, S.; Hermosilla, C. Epidemiología de la difilobotriasis en la cuenca del río Valdivia, Chile. Rev. Saude Publica 1989, 23, 45–57. [Google Scholar] [CrossRef]
- Viozzi, G.; Semenas, L.; Brugni, N.; Flores, V.R. Metazoan parasites of Galaxias maculatus (Osmeriformes: Galaxiidae) from Argentinean Patagonia. Comp. Parasitol. 2009, 76, 229–239. [Google Scholar] [CrossRef]
- Waeschenbach, A.; Brabec, J.; Scholz, T.; Littlewood, D.T.J.; Kuchta, R. The catholic taste of broad tapeworms–multiple routes to human infection. Int. J. Parasitol. 2017, 47, 831–843. [Google Scholar] [CrossRef]
- Kuchta, R.; Radačovská, A.; Bazsalovicsová, E.; Viozzi, G.; Semenas, L.; Arbetman, M.; Scholz, T. Host switching of zoonotic broad fish tapeworm (Dibothriocephalus latus) to salmonids, Patagonia. Emerg. Infect. Dis. 2019, 25, 2156–2158. [Google Scholar] [CrossRef] [Green Version]
- Ortubay, S.; Semenas, L.; Ubeda, C.; Quaggiotto, A.; Viozzi, G. Catálogo de Peces Dulceacuícolas de la Patagonia Argentina y sus Parásitos Metazoos; Dirección de Pesca de la provincia de Río Negro: Viedma, Argentina, 1994; Volume 1, p. 110. [Google Scholar]
- Rauque, C.A.; Viozzi, G.P.; Semenas, L.G. Component population study of Acanthocephalus tumescens (Acanthocephala) in fishes from Lake Moreno, Argentina. Folia Parasitol. 2003, 50, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Gil de Pertierra, A.A.; Semenas, L.G. Ailinella mirabilis gen. n., sp. n. (Eucestoda: Pseudophyllidea) from Galaxias maculatus (Pisces: Galaxiidae) in the Andean-Patagonian region of Argentina. Folia Parasitol. 2006, 53, 276–286. [Google Scholar] [CrossRef]
- Gil de Pertierra, A.A.; Semenas, L. Galaxitaenia toloi n. gen. n. sp. (Eucestoda: Pseudophyllidea) from Galaxias platei (Pisces: Osmeriformes, Galaxiidae), in the Patagonian region of Argentina. J. Parasitol. 2005, 91, 900–908. [Google Scholar] [CrossRef]
- Ostrowski de Núñez, M.; Flores, V.; Viozzi, G.; Kreiter, A. Stephanoprora uruguayense Holcman-Spector et Olague, 1989 (Digenea, Echinostomatidae) from Argentina, and comment on species of Stephanoprora from birds of the neotropical region. Acta Parasitol. 2004, 49, 292–299. [Google Scholar]
- Semenas, L.; Úbeda, C.; Ortubay, S.; Noguera, P.; Revenga, J.; Viozzi, G. Estado sanitario de las poblaciones de peces de cuerpos de agua andino patagónicos. In Actas Primeras Jornadas Nacionales de Fauna Silvestre; Uni. Nac. La Pampa: Santa Rosa, Argentina, 1987; pp. 329–347. [Google Scholar]
- Bonnett, M.L.; Lambert, P.W. Diet of giant kokopu, Galaxias argenteus. N. Z. J. Mar. Freshw. Res. 2002, 36, 361–369. [Google Scholar] [CrossRef]
- Rashnavadi, M. The Ecological Impacts of Secondary Salinisation on Halo-Tolerant Fishes in South-Western Australia. Ph.D. Thesis, Murdoch University, Perth, Australia, 2010. [Google Scholar]
- McDowall, R.M. Galaxias maculatus (Jenyns), the New Zealand Whitebait; Fisheries Research Bulletin: Wellington, New Zealand, 1968; Volume 2, p. 84. [Google Scholar]
- Llewellyn, L. Breeding biology, and egg and larval development of Galaxias rostratus Klunzinger, the Murray Jollytail from inland New South Wales. Aust. Zool. 2005, 33, 141–165. [Google Scholar] [CrossRef] [Green Version]
- Raadik, T.A. A Research Recovery Plan for the Barred Galaxias in South-Eastern Australia; Flora and Fauna Technical Report No. 141; Department of Conservation and Natural Resources: Melbourne, Australia, 1995; p. 25.
- Allibone, R.M. Water Abstraction Impacts on Non-Migratory Galaxiids of Otago Streams; Science for Conservation 147; Department of Conservation: Wellington, New Zealand, 2000; p. 43.
- O’Brien, L. The Conservation Ecology of Canterbury Mudfish (Neochanna burrowsius). Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2005. [Google Scholar]
- Johnston, T.H.; Mawson, P.M. Some nematodes parasitic in Australian freshwater fish. Trans. R. Soc. S. Aust. 1940, 64, 340–352. [Google Scholar]
- Duhig, J.V. On two fish of the species Galaxias o’connori (Ogilby) suffering from melanosis. Proc. R. Soc. Qld. 1930, xvi, 42. [Google Scholar]
- Cussac, V.; Ortubay, S.; Iglesias, G.; Milano, D.; Lattuca, M.E.; Barriga, J.P.; Battini, M.; Gross, M. The distribution of South American galaxiid fishes: The role of biological traits and post-glacial history. J. Biogeogr. 2004, 31, 103–121. [Google Scholar] [CrossRef]
- Fryer, G. A new freshwater species of the genus Dolops (Crustacea: Branchiura) parasitic on a galaxiid fish of Tasmania-with comments on disjunct distribution patterns in the southern hemisphere. Aust. J. Zool. 1969, 17, 49–64. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Available online: https://www.ala.org.au/ (accessed on 13 September 2020).
- Coleman, R.; Raadik, T.; Freeman, R. Galaxiella pusilla; The IUCN Red List of Threatened Species. 2019. Available online: https://www.researchgate.net/profile/Tarmo_Raadik/publication/340662947_Galaxiella_pusilla_Dwarf_Galaxias_The_IUCN_Red_List_of_Threatened_Species_2019/links/5e97cb6d92851c2f52a63586/Galaxiella-pusilla-Dwarf-Galaxias-The-IUCN-Red-List-of-Threatened-Species-2019.pdf (accessed on 13 February 2019). [CrossRef]
- McDowall, R.; Allibone, R.; Chadderton, W. Issues for the conservation and management of Falkland Islands freshwater fishes. Aquat. Conserv. 2001, 11, 473–486. [Google Scholar] [CrossRef]
- Keith, P. Threatened fishes of the world: Galaxias neocaledonicus Weber & De Beaufort, 1913 (Galaxiidae). Environ. Biol. Fishes 2002, 63, 26. [Google Scholar] [CrossRef]
- Crow, S. New Zealand Freshwater Fish Database; Version 1.6. Occurrence Dataset; The National Institute of Water and Atmospheric Research (NIWA): Auckland, The Netherlands, 2018; Available online: https://doi.org/10.15468/ms5iqu (accessed on 6 August 2020).
- R Computing Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Gelman, A. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 2008, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Scott, E. Observations on fishes of the family Galaxiidae: Part 1. In Papers and Proceedings of the Royal Society of Tasmania; The Royal Society of Tasmania: Tasmania, Australia, 1935; pp. 85–112. [Google Scholar]
- Percival, E. A note on the life history of Diplodon lutulentus Gould. Trans. Proc. R. Soc. N. Z. Inst. 1931, 62, 86–91. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.4.7). 2020. Available online: https://github.com/rvlenth/emmeans (accessed on 18 September 2020).
- Alama-Bermejo, G.; Viozzi, G.; Waicheim, M.; Flores, V.; Atkinson, S. Host-parasite relationship of Ortholinea lauquen sp. nov. (Cnidaria: Myxozoa) and the fish Galaxias maculatus in northwestern Patagonia, Argentina. Dis. Aquat. Organ. 2019, 136, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Hoffmann, A. Digenean trematode cysts within the heads of threatened Galaxiella species (Teleostei: Galaxiidae) from south-eastern Australia. Aust. J. Zool. 2016, 64, 285–291. [Google Scholar] [CrossRef]
- McCredden, M. Anchors Away: The Susceptibility and Response to Infection between Native and Co-Introduced Fishes to the Alien Anchor Worm Lernaea cyprinacea. Ph.D. Thesis, Murdoch University, Perth, Australia, 2016. [Google Scholar]
- Luque, J.L.; Vieira, F.M.; Herrmann, K.; King, T.M.; Poulin, R.; Lagrue, C. New evidence on a cold case: Trophic transmission, distribution and host-specificity in Hedruris spinigera (Nematoda: Hedruridae). Folia Parasitol. 2010, 57, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, F.; White, R.S.A.; Paterson, R.A. Hiding in the swamp: New capillariid nematode parasitizing New Zealand brown mudfish. J. Helminthol. 2018, 92, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cribb, T.; Bray, R.; Wright, T.; Pichelin, S. The trematodes of groupers (Serranidae: Epinephelinae): Knowledge, nature and evolution. Parasitology 2002, 124, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.; Takemoto, R.; Pavanelli, G.; Adriano, E. About the biodiversity of parasites of freshwater fish from Brazil. Bull. Eur. Assoc. Fish Pathol. 2011, 31, 161–168. [Google Scholar]
- Chaganti, K.; Brickle, P.; Mackenzie, K. Two new species of myxozoan parasites (Myxosporea, Multivalvulida, Bivalvulida) from fishes of the Falkland Islands. Acta Parasitol. 2000, 45, 285–288. [Google Scholar]
- Cussac, V.E.; Habit, E.; Ciancio, J.; Battini, M.A.; Riva Rossi, C.; Barriga, J.P.; Baigún, C.; Crichigno, S. Freshwater fishes of Patagonia: Conservation and fisheries. J. Fish Biol. 2016, 89, 1068–1097. [Google Scholar] [CrossRef]
- Dyer, B.S. Systematic review and biogeography of the freshwater fishes of Chile revision sistematica y biogeografica de los peces dulceacuicolas de Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Raadik, T.A. Fifteen from one: A revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 2014, 3898, 1–198. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.M.; Rowe, D.L.; Burridge, C.P.; Wallis, G.P. Gene trees versus species trees: Reassessing life-history evolution in a freshwater fish radiation. Syst. Biol. 2010, 59, 504–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakona, G.; Swartz, E.R.; Chakona, A. The status and distribution of a newly identified endemic galaxiid in the eastern Cape Fold Ecoregion, of South Africa. Aquat. Conserv. 2018, 28, 55–67. [Google Scholar] [CrossRef]
- Morand, S. Wormy world: Comparative tests of theoretical hypotheses on parasite species richness. In Evolutionary Biology of Host–Parasite Relationships: Theory Meets Reality; Poulin, R., Morand, S., Skorping, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 63–79. [Google Scholar]
- Lo, C.M.; Morand, S.; Galzin, R. Parasite diversity\host age and size relationship in three coral-reef fishes from French Polynesia. Int. J. Parasitol. 1998, 28, 1695–1708. [Google Scholar] [CrossRef]
- Chen, H.-W.; Liu, W.-C.; Davis, A.J.; Jordán, F.; Hwang, M.-J.; Shao, K.-T. Network position of hosts in food webs and their parasite diversity. Oikos 2008, 117, 1847–1855. [Google Scholar] [CrossRef]
- Rojo, J.H.; Figueroa, D.E.; Boy, C.C. Age and growth of diadromous Galaxias maculatus (Jenyns, 1842) in southernmost South America (54° S) including contribution of age classes to reproduction. Environ. Biol. Fish. 2018, 101, 1149–1160. [Google Scholar] [CrossRef]
- Fernández, M.; Semenas, L.; Viozzi, G. La estructura de las comunidades de helmintos de Galaxias maculatus (Osmeriformes: Galaxiidae) en diferentes sitios de un lago de la Patagonia argentina. Ecol. Austral 2015, 25, 212–220. [Google Scholar] [CrossRef]
- Cirtwill, A.R.; Stouffer, D.B.; Poulin, R.; Lagrue, C. Are parasite richness and abundance linked to prey species richness and individual feeding preferences in fish hosts? Parasitology 2016, 143, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshw. Fish 2014, 23, 615–621. [Google Scholar] [CrossRef]
- McDowall, R.M. New Zealand Freshwater Fishes: A Natural History and Guide; Heinemann Reed: Auckland, New Zealand, 1990. [Google Scholar]
- Herrmann, K.K.; Poulin, R.; Keeney, D.B.; Blasco-Costa, I. Genetic structure in a progenetic trematode: Signs of cryptic species with contrasting reproductive strategies. Int. J. Parasitol. 2014, 44, 811–818. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Flores, V.; Viozzi, G. Redescription, seasonality and distribution of Myxobolus magellanicus (Myxosporea) in Galaxias maculatus (Osmeriformes, Galaxiidae) from Patagonian Andean lakes (Argentina). Acta Parasitol. 2001, 46, 159–163. [Google Scholar]
- Flores, V.; Viozzi, G. Infection of Myxobolus galaxii (Myxozoa) in Galaxias maculatus (Osmeriformes: Galaxiidae) from Northwestern Patagonian Andean Lakes (Argentina). J. Parasitol. 2007, 93, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Viozzi, G.P.; Flores, V.R. Myxidium biliare sp. n. (Myxozoa) from gall bladder of Galaxias maculatus (Osmeriformes: Galaxiidae) in Patagonia (Argentina). Folia Parasitol. 2003, 50, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, R.; Jorge, F. The geography of parasite discovery across taxa and over time. Parasitology 2019, 146, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Noia, M.; Poole, R.; Kaufmann, J.; Waters, C.; Adams, C.; McGinnity, P.; Llewellyn, M. In-situ non-lethal rapid test to accurately detect the presence of the nematode parasite, Anguillicoloides crassus, in European eel, Anguilla anguilla. bioRxiv 2020. [Google Scholar] [CrossRef]
- Berger, C.S.; Aubin-Horth, N. An eDNA-qPCR assay to detect the presence of the parasite Schistocephalus solidus inside its three spine stickleback host. J. Exp. Biol. 2018, 221, jeb178137. [Google Scholar] [CrossRef] [Green Version]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]
- Kvach, Y.; Ondračková, M.; Janáč, M.; Jurajda, P. Methodological issues affecting the study of fish parasites. III. Effect of fish preservation method. Dis. Aquat. Organ. 2018, 127, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Viozzi, G.P.; Marín, S.L.; Carvajal, J.; Brugni, N.; Mancilla, M. A new genus of dactylogyrid from the gills of Galaxias maculatus (Osmeriformes: Galaxiidae) in Maullín basin, Patagonia, Chile. J. Parasitol. 2007, 93, 542–544. [Google Scholar] [CrossRef]
- Viozzi, G.; Flores, V.; Marín, S.L.; Mancilla, M.; Carvajal, J. Parasites of the Red Jollytail, Brachygalaxias bullocki (Osmeriformes: Galaxiidae), from the Maullín River, Patagonia, Chile. Comp. Parasitol. 2008, 75, 326–328. [Google Scholar] [CrossRef]
- O’Brien, L.K.; Dunn, N.R. Mudfish (Neochanna Galaxiidae) Literature Review; Sci. Conserv. 277; Science & Technical Publication, Department of Conservation: Wellington, New Zealand, 2007.
- Rauque, C.; Viozzi, G.; Flores, V.; Vega, R.; Waicheim, A.; Salgado-Maldonado, G. Helminth parasites of alien freshwater fishes in Patagonia (Argentina). Int. J. Parasitol. Parasites Wildl. 2018, 7, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.L.; Heath, A.C.; Cardoso, P. Methods for the assessment and conservation of threatened animal parasites. Biol. Conserv. 2020, 248, 108696. [Google Scholar] [CrossRef]




| Geographic Region | Galaxiidae Species | N Studies | N Fish a | N Locations | Max. Standard Length (cm) | Latitudinal Range Size (km) | N Taxa (Macro/Micro) | Taxonomic Resolution | N Molecular e | Examination f | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Genus | Family | Order | Class | Phylum | Full Body | Focal Tissue | Single Species | Incidental | |||||||||
| Argentina | Aplochiton zebra | 3 | 254 | 5 | 27.8 | 1581.4 | 18 (15/3) | 11 | 7 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Galaxias maculatus | 48 | 22,073 | 63 | 19.0 | 1742.9 | 37 (30/6) c | 24 | 10 | 3 | 0 | 0 | 0 | 1 | 9 | 5 | 33 | 1 | |
| Galaxias platei | 13 | 239 | 22 | 30.9 | 1737.1 | 22 (22/0) | 8 | 6 | 6 | 1 | 0 | 0 | 0 | 3 | 0 | 10 | 0 | |
| Australia | Galaxias auratus | 3 | 59 | 2 | 24.0 | 25.8 | 4 (4/0) | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Galaxias brevipinnis | 1 | unknown | 1 | 28.0 | 1475.8 | 2 (1/1) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Galaxias fontanus | - | - | - | 13.5 | 71.0 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias fuscus | 1 | unknown | 1 | 12.3 | 63.3 | 1 (1/0) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Galaxias johnstoni | 1 | unknown | 1 | 14.0 | 111.2 | 1 (1/0) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Galaxias maculatus | 12 | 4517 | 16 | 19.0 | 1792.8 | 12 (11/1) | 7 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 10 | 0 | |
| Galaxias niger | - | - | - | 10.5 | 1.11 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias occidentalis | 6 | 1773 | 16 | 19.0 | 600.4 | 8 (8/0) | 2 | 4 | 1 | 0 | 1 | 0 | 2 | 1 | 0 | 5 | 0 | |
| Galaxias olidus | 8 | 3066 | 7 | 15.0 | 1591.8 | 9 (7/2) | 7 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 7 | 0 d | |
| Galaxias parvus | - | - | - | 10.0 | 31.7 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias pedderensis | - | - | - | 16.0 | 43.7 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias rostratus | 1 | 1 | 2 | 12.0 | 861.7 | 2 (1/0) c | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Galaxias tanycephalus | - | - | - | 14.5 | 17.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias truttaceus | 2 | 653 | 2 | 20.0 | 1083.0 | 4 (4/0) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | |
| Galaxiella munda | - | - | - | 6.0 | 389.2 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxiella nigrostriatab | 1 | 2266 | 4 | 5.0 | 177.9 | 0 | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxiella pusilla | 1 | 38 | 2 | 4.8 | 527.7 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Galaxiella toourtkoourt | 1 | 120 | 4 | 3.1 | 245.8 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Lovettia sealii | 1 | 12 | 1 | 7.2 | 540.7 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Neochanna cleaveri | - | - | - | 12.5 | 719.4 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Paragalaxias dissimilis | - | - | - | 7.5 | 39.6 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Paragalaxias eleotroides | - | - | - | 5.9 | 113.4 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Paragalaxias julianus | - | - | - | 10.0 | 61.2 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Paragalaxias mesotes | - | - | - | 8.0 | 40.5 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Chile | Aplochiton marinus | - | - | - | unknown | <1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Aplochiton taeniatus | 1 | 3 | 1 | 33.4 | 1738.3 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Aplochiton zebra | 2 | 4 | 3 | 27.8 | 1780.9 | 2 (2/0) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | |
| Brachygalaxias bullocki | 3 | 60 | 3 | 5.5 | 643.0 | 9 (4/5) | 4 | 4 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | |
| Brachygalaxias gothei | - | - | - | unknown | <1 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias globiceps | - | - | - | 10.7 | 137.1 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias maculatus | 14 | 1346 | 26 | 19.0 | 2496.2 | 23 (20/3) | 11 | 9 | 2 | 1 | 0 | 0 | 0 | 2 | 3 | 9 | 0 | |
| Galaxias platei | 4 | 81 | 5 | 30.9 | 1847.6 | 5 (5/0) | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | |
| Islas Malvinas/ | Aplochiton zebra | 1 | 30 | 1 | 27.8 | 68.9 | 1 (1/0) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Falkland Islands | Galaxias maculatus | 1 | 6 | 1 | 19.0 | 93.4 | 1 (0/1) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Galaxias platei | - | - | - | unknown | unknown | - | - | - | - | - | - | - | - | - | - | - | - | |
| New Caledonia | Galaxias neocaledonicus | - | - | - | 16.6 | 1.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| New Zealand | Galaxias anomalus | 4 | 743 | 6 | 6.1 | 111.7 | 7 (7/0) | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 |
| Galaxias argenteus | 2 | 114 | 2 | 34.0 | 173.4 | 4 (4/0) | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Galaxias brevipinnis | 12 | 36 | 10 | 28.0 | 1319.9 | 13 (11/2) | 7 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 4 | 4 | 0 d | |
| Galaxias cobitinis | - | - | - | 7.0 | 142.3 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias depressiceps | 1 | 51 | 4 | 7.3 | 129.5 | 2 (2/0) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Galaxias divergens | 2 | unknown | 2 | 6.0 | 542.4 | 4 (4/0) | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 d | |
| Galaxias eldoni | - | - | - | 7.5 | 47.8 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias fasciatus | 1 | unknown | 1 | 21.5 | 1423.9 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 d | |
| Galaxias gollumoides | 1 | 7 | 1 | 17.2 | 249.1 | 2 (2/0) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Galaxias gracilis | - | - | - | 6.2 | 80.3 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias macronasus | - | - | - | 7.0 | 99.3 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias maculatus | 20 | 989 | 11 | 19.0 | 1424.5 | 18 (17/1) | 9 | 4 | 0 | 3 | 2 | 0 | 1 | 1 | 4 | 12 | 1 d | |
| Galaxias paucispondylus | - | - | - | 8.8 | 468.7 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias postvectis | 1 | unknown | 1 | 26.0 | 1229.1 | 1 (1/0) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 d | |
| Galaxias prognathus | - | - | - | 8.0 | 213.5 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias pullus | - | - | - | 13.1 | 50 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Galaxias vulgaris | 4 | 52 | 4 | 8.9 | 374.7 | 3 (2/0) c | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 d | |
| Neochanna apoda | 4 | 312 | 8 | 9.6 | 450.8 | 8 (6/1) c | 3 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | |
| Neochanna burrowsius | 1 | 1091 | 4 | 13.3 | 218.9 | 4 (0/2) c | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Neochanna diversus | 1 | unknown | 1 | 12.2 | 434.9 | 1 (1/0) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 d | |
| Neochanna heleios | - | - | - | 10.5 | 28.4 | - | - | - | - | - | - | - | - | - | - | - | - | |
| Neochanna rekohua | - | - | - | 6.6 | 4.7 | - | - | - | - | - | - | - | - | - | - | - | - | |
| South Africa | Galaxias zebratus | - | - | - | 6.8 | 444.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| Df | Deviance | Residual Df | Residual Deviance | F | p | |
|---|---|---|---|---|---|---|
| NULL | 32 | 251.20 | ||||
| N studies | 1 | 134.32 | 31 | 116.88 | 423.59 | <0.001 |
| Region | 3 | 20.95 | 28 | 95.93 | 22.03 | <0.001 |
| Fish length | 1 | 1.44 | 27 | 94.49 | 4.54 | 0.050 |
| Range size | 1 | 21.79 | 26 | 72.70 | 68.73 | <0.001 |
| N studies × Region | 3 | 49.95 | 23 | 22.75 | 52.51 | <0.001 |
| N studies × Fish length | 1 | 0.47 | 22 | 22.28 | 1.47 | 0.245 |
| N studies × Range size | 1 | 9.49 | 21 | 12.79 | 29.93 | <0.001 |
| Region × Fish length | 3 | 2.61 | 18 | 10.18 | 2.74 | 0.080 |
| Region × Range size | 2 | 3.81 | 16 | 6.37 | 6.01 | 0.012 |
| Fish length × Range size | 1 | 1.39 | 15 | 4.98 | 4.39 | 0.054 |
| NULL | 24 | 172.76 | NA | NA | ||
| N fish | 1 | 67.68 | 23 | 105.07 | 40.21 | <0.001 |
| Region | 3 | 40.13 | 20 | 64.94 | 7.95 | 0.012 |
| Fish length | 1 | 3.79 | 19 | 61.15 | 2.25 | 0.177 |
| Range size | 1 | 20.98 | 18 | 40.17 | 12.46 | 0.010 |
| N fish × Region | 3 | 18.44 | 15 | 21.73 | 3.65 | 0.072 |
| N fish × Fish length | 1 | 0.56 | 14 | 21.17 | 0.33 | 0.582 |
| N fish × Range size | 1 | 3.11 | 13 | 18.06 | 1.85 | 0.216 |
| Region × Fish length | 3 | 0.67 | 10 | 17.39 | 0.13 | 0.938 |
| Region × Range size | 2 | 3.79 | 8 | 13.60 | 1.13 | 0.376 |
| Fish length × Range size | 1 | 1.91 | 7 | 11.69 | 1.14 | 0.322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterson, R.A.; Viozzi, G.P.; Rauque, C.A.; Flores, V.R.; Poulin, R. A Global Assessment of Parasite Diversity in Galaxiid Fishes. Diversity 2021, 13, 27. https://doi.org/10.3390/d13010027
Paterson RA, Viozzi GP, Rauque CA, Flores VR, Poulin R. A Global Assessment of Parasite Diversity in Galaxiid Fishes. Diversity. 2021; 13(1):27. https://doi.org/10.3390/d13010027
Chicago/Turabian StylePaterson, Rachel A., Gustavo P. Viozzi, Carlos A. Rauque, Verónica R. Flores, and Robert Poulin. 2021. "A Global Assessment of Parasite Diversity in Galaxiid Fishes" Diversity 13, no. 1: 27. https://doi.org/10.3390/d13010027
APA StylePaterson, R. A., Viozzi, G. P., Rauque, C. A., Flores, V. R., & Poulin, R. (2021). A Global Assessment of Parasite Diversity in Galaxiid Fishes. Diversity, 13(1), 27. https://doi.org/10.3390/d13010027