Seagrass Patch Complexity Affects Macroinfaunal Community Structure in Intertidal Areas: An In Situ Experiment Using Seagrass Mimics
Abstract
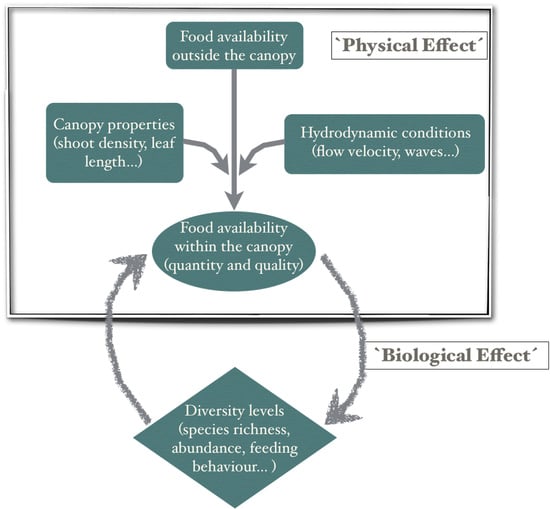
:1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Mimics Design
2.3. In Situ Experimental Set-Up
2.4. Sampling Procedure
2.5. Analytical Methods
2.6. Carbon and Nitrogen Composition and Isotopic Analysis
2.7. Statistics
3. Results
3.1. Effects of Canopy Properties on Species Richness and Abundance
3.2. Effects of Canopy Properties on Scrobicularia Plana and Hediste Diversicolor Abundance
3.3. Spatial Explicit Gradients on S. plana and H. diversicolor Distribution within the Canopy
3.4. Organic Matter Content, Epiphytes, and Egg Masses
3.5. Deriving Diet Shifts from Stable Isotopes
4. Discussion
4.1. Response of Macrobenthic Community
4.2. Shifts of Diets for Macrobenthic Fauna
4.3. Ecological Significance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Vassallo, P.; Paoli, C.; Rovere, A.; Montefalcone, M.; Morri, C.; Bianchi, C.N. The value of the seagrass Posidonia oceanica: A natural capital assessment. Mar. Pol. Bull. 2013, 75, 157–167. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: New York, NY, USA, 2000; 310p. [Google Scholar] [CrossRef]
- Boström, C.; O’Brien, K.; Roos, C.; Ekebom, J. Environmental variables explaining structural and functional diversity of seagrass macrofauna in an archipelago landscape. J. Exp. Mar. Biol. Ecol. 2006, 335, 52–73. [Google Scholar] [CrossRef]
- Larkum, A.W.D.; Orth, R.J.; Duarte, C.M. (Eds.) Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; 691p. [Google Scholar] [CrossRef]
- Duffy, J.E. Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol. Prog. Ser. 2006, 311, 233–250. [Google Scholar] [CrossRef] [Green Version]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 739–766. [Google Scholar] [CrossRef] [Green Version]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrush, S.F.; Dayton, P.K. Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu. Rev. Ecol. Syst. 2002, 33, 449–473. [Google Scholar] [CrossRef] [Green Version]
- Norkko, A.; Hewitt, J.E.; Thrush, S.F.; Funnell, G.A. Conditional outcomes of facilitation by a habitat-modifying subtidal bivalve. Ecology 2006, 87, 226–234. [Google Scholar] [CrossRef]
- Vogel, S. Life in Moving Fluids; Willard Grant: Boston, MA, USA, 1981; 352p. [Google Scholar]
- Kuo, J.; den Hartog, C. Seagrass morphology, anatomy, and ultrastructure. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 51–87. [Google Scholar] [CrossRef]
- Hovel, K.A.; Lipcius, R.N. Effects of seagrass habitat fragmentation on juvenile blue crab survival and abundance. J. Exp. Mar. Biol. Ecol. 2002, 271, 75–98. [Google Scholar] [CrossRef]
- Holmquist, J.G. Permeability of patch boundaries to benthic invertebrates: Influences of boundary contrast, light level, and faunal density and mobility. Oikos 1998, 81, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Hovel, K.A.; Regan, H.M. Using an individual-based model to examine the roles of habitat fragmentation and behavior on predator–prey relationships in seagrass landscapes. Landsc. Ecol. 2008, 23, 75–89. [Google Scholar] [CrossRef]
- González-Ortiz, V.; Egea, L.G.; Jiménez-Ramos, R.; Moreno-Marín, F.; Pérez-Lloréns, J.L.; Bouma, T.J.; Brun, F.G. Interactions between seagrass complexity, hydrodynamic flow and biomixing alter food availability for associated filter-feeding organisms. PLoS ONE 2014, 9, e104949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduin, J.J.; Backhaus, J.O. Dynamics of plant–flow interactions for the seagrass Amphibolis antarctica: Field observations and model simulations. Estuar. Coast. Shelf Sci. 2000, 50, 185–204. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Low, E.; Peralta, G.; Tánczos, C.; Van de Koppel, J.; Herman, P.M.J. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology 2005, 86, 2187–2199. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.P.; Peralta, G.; Brun, F.G.; van Duren, L.A.; Bouma, T.J.; Pérez-Lloréns, J.L. Interaction between hydrodynamics and seagrass canopy structure: Spatially explicit effects on ammonium uptake rates. Limnol. Oceanogr. 2008, 53, 1531–1539. [Google Scholar] [CrossRef]
- Irlandi, E.A.; Peterson, C.H. Modification of animal habitat by large plants: Mechanisms by which seagrasses influence clam growth. Oecologia 1991, 87, 307–318. [Google Scholar] [CrossRef]
- Peterson, B.J.; Heck, K.L., Jr. Positive interactions between suspension-feeding bivalves and seagrass - a facultative mutualism. Mar. Ecol. Prog. Ser. 2001, 213, 143–155. [Google Scholar] [CrossRef]
- Brun, F.G.; Van Zetten, E.; Cacabelos, E.; Bouma, T.J. Role of two contrasting ecosystem engineers (Zostera noltii and Cymodocea nodosa) on the food intake rate of Cerastoderma edule. Helgol. Mar. Res. 2009, 63, 19–25. [Google Scholar] [CrossRef]
- Tagliapietra, D.; Pessa, G.; Cornello, M.; Zitelli, A.; Magni, P. Temporal distribution of intertidal macrozoobenthic assemblages in a Nanozostera noltii-dominated area (Lagoon of Venice). Mar. Environ. Res. 2016, 114, 31–39. [Google Scholar] [CrossRef]
- Jiménez-Ramos, R.; Egea, L.G.; Vergara, J.J.; Bouma, T.J.; Brun, F.G. The role of flow velocity combined with habitat complexity as a top–down regulator in seagrass meadows. Oikos 2019, 128, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Rodil, I.F.; Lohrer, A.M.; Attard, K.M.; Hewitt, J.E.; Thrush, S.F.; Norkko, A. Macrofauna communities across a seascape of seagrass meadows: Environmental drivers, biodiversity patterns and conservation implications. Biodivers. Conserv. 2021, 30, 3023–3043. [Google Scholar] [CrossRef]
- Attrill, M.J.; Strong, J.A.; Rowden, A.A. Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 2000, 23, 114–121. [Google Scholar] [CrossRef]
- Hovel, K.A.; Fonseca, M.S. Influence of seagrass landscape structure on the juvenile blue crab habitat-survival function. Mar. Ecol. Prog. Ser. 2005, 300, 170–191. [Google Scholar] [CrossRef]
- Sirota, L.; Hovel, K.A. Simulated eelgrass Zostera marina structural complexity: Effects of shoot length, shoot density, and surface area on the epifaunal community of San Diego Bay, California, USA. Mar. Ecol. Prog. Ser. 2006, 326, 115–131. [Google Scholar] [CrossRef]
- Gagnon, K.; Rinde, E.; Bengil, E.G.T.; Carugati, L.; Christianen, M.J.A.; Danovaro, R.; Gambi, C.; Govers, L.L.; Kipston, S.; Meysick, L.; et al. Facilitating foundation species: The potential for plant–bivalve interactions to improve habitat restoration success. J. Appl. Ecol. 2020, 57, 1161–1179. [Google Scholar] [CrossRef]
- Barbier, P.; Meziane, T.; Forêt, M.; Tremblay, R.; Robert, R.; Olivier, F. Nursery function of coastal temperate benthic habitats: New insight from the bivalve recruitment perspective. J. Sea Res. 2017, 121, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Peralta, G.; Morris, E.P.; van Duren, L.A.; Bouma, T.J. Consequences of shoot density and stiffness for ecosystem engineering by benthic macrophytes in flow dominated areas: A hydrodynamic flume study. Mar. Ecol. Prog. Ser. 2008, 368, 103–115. [Google Scholar] [CrossRef] [Green Version]
- González-Ortiz, V.; Alcazar, P.; Vergara, J.J.; Pérez-Lloréns, J.L.; Brun, F.G. Effects of two antagonistic ecosystem engineers on infaunal diversity. Estuar. Coast. Shelf Sci. 2014, 139, 20–26. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Williams, S.L. Macrophyte canopy structure and the success of an invasive marine bivalve. Oikos 1999, 84, 398–416. [Google Scholar] [CrossRef]
- Allen, B.J.; Williams, S.L. Native eelgrass Zostera marina controls growth and reproduction of an invasive mussel through food limitation. Mar. Ecol. Prog. Ser. 2003, 254, 57–67. [Google Scholar] [CrossRef]
- Tsai, C.; Yang, S.; Trimble, A.C.; Ruesink, J.L. Interactions between two introduced species: Zostera japonica (dwarf eelgrass) facilitates itself and reduces condition of Ruditapes philippinarum (Manila clam) on intertidal flats. Mar. Biol. 2010, 157, 1929–1936. [Google Scholar] [CrossRef]
- Carroll, J.M.; Peterson, B.J. Ecological trade-offs in seascape ecology: Bay scallop survival and growth across a seagrass seascape. Landsc. Ecol. 2013, 28, 1401–1413. [Google Scholar] [CrossRef]
- Brun, F.G.; Cummaudo, F.; Olivé, I.; Vergara, J.J.; Pérez-Lloréns, J.L. Clonal extent, apical dominance and networking features in the phalanx angiosperm Zostera noltii Hornem. Mar. Biol. 2007, 151, 1917–1927. [Google Scholar] [CrossRef] [Green Version]
- Brun, F.G.; Olivé, I.; Malta, E.-J.; Vergara, J.J.; Hernández, I.; Pérez-Lloréns, J.L. Increased vulnerability of Zostera noltii to stress caused by low light and elevated ammonium levels under phosphate deficiency. Mar. Ecol. Prog. Ser. 2008, 365, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Goshima, S.; Peterson, C. Both below- and aboveground shoalgrass structure influence whelk predation on hard clams. Mar. Ecol. Prog. Ser. 2012, 451, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Barrón, C.; Middelburg, J.J.; Duarte, C.M. Phytoplankton trapped within seagrass (Posidonia oceanica) sediments are a nitrogen source: An in situ isotope labelling experiment. Limnol. Oceanogr. 2006, 51, 1648–1653. [Google Scholar] [CrossRef] [Green Version]
- Barcelona, A.; Oldham, C.; Colomer, J.; Garcia-Orellana, J.; Serra, T. Particle capture by seagrass canopies under an oscillatory flow. Coast. Eng. 2021, 169, 103972. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Williams, S.L. Variable responses of native eelgrass Zostera marina to a non-indigenous bivalve Musculista senhousia. Oecologia 1998, 113, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Bouma, T.J.; Ortells, V.; Ysebaert, T. Comparing biodiversity effects among ecosystem engineers of contrasting strength: Macrofauna diversity in Zostera noltii and Spartina anglica vegetations. Helgol. Mar. Res. 2009, 63, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Giraldo-Ospina, A.; Ladah, L.B.; Hovel, K.A. Changes in within-canopy environmental conditions and the population structure of the speckled scallop associated to localized loss of above-ground seagrass cover. J. Exp. Mar. Biol. Ecol. 2021, 534, 151486. [Google Scholar] [CrossRef]
- Self, R.F.L.; Jumars, P.A. Cross-phyletic patterns of particle selection by deposit feeders. J. Mar. Res. 1988, 46, 119–143. [Google Scholar] [CrossRef]
- Defossez, J.-M.; Hawkins, A.J.S. Selective feeding in shellfish: Size-dependent rejection of large particles within pseudofaeces from Mytilus edulis, Ruditapes philippinarum and Tapes decussatus. Mar. Biol. 1997, 129, 139–147. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Effects of increasing current velocity, turbidity and particle-size selection on the feeding activity and scope for growth of Ruditapes decussatus from Ria Formosa, southern Portugal. J. Exp. Mar. Biol. Ecol. 2000, 245, 111–125. [Google Scholar] [CrossRef]
- Brun, F.G.; Pérez-Lloréns, J.L.; Hernández, I.; Vergara, J.J. Patch distribution and within-patch dynamics of the seagrass Zostera noltii Hornem. in Los Toruños Salt-Marsh, Cádiz Bay Natural park, Spain. Bot. Mar. 2003, 46, 513–524. [Google Scholar] [CrossRef]
- González-Gordillo, J.I.; Arias, A.M.; Rodríguez, A.; Drake, P. Recruitment patterns of decapod crustacean megalopae in a shallow inlet (SW Spain) related to life history strategies. Estuar. Coast. Shelf Sci. 2003, 56, 593–607. [Google Scholar] [CrossRef]
- Ferrón, S.; Ortega, T.; Forja, J.M. Benthic fluxes in a tidal salt marsh creek affected by fish farm activities: Río San Pedro (Bay of Cádiz, SW Spain). Mar. Chem. 2009, 113, 50–62. [Google Scholar] [CrossRef]
- Brun, F.G.; González-Ortiz, V.; Vergara, J.J.; Pérez-Lloréns, J.L. Unidad artificial flexible individual de angiosperma marina. Flexible artificial single seagrass unit. National Patent 201200489, 7 May 2012. [Google Scholar]
- Brun, F.G.; Vergara, J.J.; Peralta, G.; García-Sánchez, M.P.; Hernández, I.; Pérez- Lloréns, J.L. Clonal building, simple growth rules and phylloclimate as key steps to develop functional-structural seagrass models. Mar. Ecol. Prog. Ser. 2006, 323, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Merino, M.; Agawin, N.S.; Uri, J.; Fortes, M.D.; Gallegos, M.E.; Marbá, N.; Hemminga, M.A. Root production and belowground seagrass biomass. Mar. Ecol. Prog. Ser. 1998, 171, 97–108. [Google Scholar] [CrossRef]
- Hughes, R.N. Population Dynamics of the Bivalve Scrobicularia plana (Da Costa) on an Intertidal Mud-Flat in North Wales. J. Anim. Ecol. 1970, 39, 333–356. [Google Scholar] [CrossRef]
- Jacobs, R.P.W.M.; Hegger, H.H.; Ras-Willems, A. Seasonal variations in the structure of a Zostera community on tidal flats in the SW Netherlands, with special reference to the benthic fauna. Proc. Kon. Ned. Akad. Wet. Ser. C 1983, 86, 347–375. [Google Scholar]
- Boström, C.; Bonsdorff, E. Community structure and spatial variation of benthic invertebrates associated with Zostera marina (L.) beds in the northern Baltic Sea. J. Sea Res. 1997, 37, 153–166. [Google Scholar] [CrossRef]
- Carabel, S.; Godínez-Domínguez, E.; Verísimo, P.; Fernández, L.; Freire, J. An assessment of sample processing methods for stable isotope analyses of marine food webs. J. Exp. Mar. Biol. Ecol. 2006, 336, 254–261. [Google Scholar] [CrossRef]
- Skilleter, G.A.; Pryor, A.; Miller, S.; Cameron, B. Detecting the effects of physical disturbance on benthic assemblages in a subtropical estuary: A Beyond BACI approach. J. Exp. Mar. Biol. Ecol. 2006, 338, 271–287. [Google Scholar] [CrossRef]
- Kanaya, G.; Takagi, S.; Nobata, E.; Kikuchi, E. Spatial dietary shift of macrozoobenthos in a brackish lagoon revealed by carbon and nitrogen stable isotope ratios. Mar. Ecol. Prog. Ser. 2007, 345, 117–127. [Google Scholar] [CrossRef]
- Kanaya, G.; Takagi, S.; Kikuchi, E. Dietary contribution of the microphytobenthos to infaunal deposit feeders in an estuarine mudflat in Japan. Mar. Biol. 2008, 155, 543–553. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual. Version 3.1. 2016. Available online: https://github.com/brianstock/MixSIAR (accessed on 7 September 2021).
- Davoult, D.; Surget, G.; Stiger-Pouvreau, V.; Noisette, F.; Riera, P.; Stagnol, D.; Androuin, T.; Poupart, N. Multiple effects of a Gracilaria vermiculophylla invasion on estuarine mudflat functioning and diversity. Mar. Environ. Res. 2017, 131, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Varela, J.L.; Spares, A.D.; Stokesbury, M.J. Feeding ecology of Atlantic bluefin tuna (Thunnus thynnus) in the Gulf of Saint Lawrence, Canada. Mar. Environ. Res. 2020, 161, 105087. [Google Scholar] [CrossRef]
- Kruskal, J.B. Nonmetric multidimensional scaling: A numerical method. Psychometrika 1964, 29, 115–129. [Google Scholar] [CrossRef]
- Shepard, R.N. The analysis of proximities: Multidimensional scaling with an unknown distance function, I. Psychometrika 1962, 27, 125–140. [Google Scholar] [CrossRef]
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Ltd: Plymouth, UK, 2001; 176p. [Google Scholar]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.P.; Jones, C.G. The concept of organisms as ecosystem engineers ten years on: Progress, limitations, and challenges. Bioscience 2006, 56, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Bouma, T.J.; Olenin, S.; Reise, K.; Ysebaert, T. Ecosystem engineering and biodiversity in coastal sediments: Posing hypotheses. Helgol. Mar. Res. 2009, 63, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Heck, K.L.; Orth, R.J., Jr. Predation in seagrass beds. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 537–550. [Google Scholar] [CrossRef]
- Bowden, D.A.; Rowden, A.A.; Attrill, M.J. Effect of patch size and in-patch location on the infaunal macroinvertebrate assemblages of Zostera marina seagrass beds. J. Exp. Mar. Biol. Ecol. 2001, 259, 133–154. [Google Scholar] [CrossRef]
- Pérez-Hurtado, A. Ecología Alimentaria de aves Limícolas Invernantes en la Bahía de Cádiz. Distribución y uso del Hábitat. Ph.D. Thesis, University of Seville, Seville, Spain, 1992. Available online: https://idus.us.es/handle/11441/79650 (accessed on 7 September 2021).
- Meysick, L.; Ysebaert, T.; Jansson, A.; Montserrat, F.; Valanko, S.; Villnäs, A.; Boström, C.; Norkko, J.; Norkko, A. Context-dependent community facilitation in seagrass meadows along a hydrodynamic stress gradient. J. Sea Res. 2019, 150, 8–23. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Hindell, J.S.; Keough, M.J.; Jenkins, G.P.; Connolly, R.M. Resource distribution influences positive edge effects in a seagrass fish. Ecology 2010, 91, 2013–2021. [Google Scholar] [CrossRef]
- Agawin, N.S.R.; Duarte, C.M. Evidence of direct particle trapping by a tropical seagrass meadow. Estuaries 2002, 25, 1205–1209. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Sintes, T.; Bouma, T.J.; Duarte, C.M. Experimental assessment and modeling evaluation of the effects of the seagrass Posidonia oceanica on flow and particle trapping. Mar. Ecol. Prog. Ser. 2008, 356, 163–173. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Bouma, T.J.; Morris, E.P.; Duarte, C.M. Effects of seagrasses and algae of the Caulerpa family on hydrodynamics and particle-trapping rates. Mar. Biol. 2010, 157, 473–481. [Google Scholar] [CrossRef]
- Rossi, F.; Baeta, A.; Marques, J.C. Stable isotopes reveal habitat-related diet shifts in facultative deposit-feeders. J. Sea Res. 2015, 95, 172–179. [Google Scholar] [CrossRef]
- Jones, A.G.; Dubois, S.F.; Desroy, N.; Fournier, J. Intertidal ecosystem engineer species promote benthic-pelagic coupling and diversify trophic pathways. Mar. Ecol. Prog. Ser. 2021, 660, 119–139. [Google Scholar] [CrossRef]
- Arias, A.M.; Drake, P. Distribution and production of the polychaete Nereis diversicolor in a shallow coastal lagoon in the Bay of Cadiz (SW Spain). Cah. Biol. Mar. 1995, 36, 201–210. [Google Scholar] [CrossRef]
- Rosa, S.; Granadeiro, J.P.; Vinagre, C.; França, S.; Cabral, H.N.; Palmeirim, J.M. Impact of predation on the polychaete Hediste diversicolor in estuarine intertidal flats. Estuar. Coast. Shelf Sci. 2008, 78, 655–664. [Google Scholar] [CrossRef]
- Mattila, J.; Heck, K.L., Jr.; Millstein, E.; Miller, E.; Gustafsson, C.; Williams, S.; Byron, D. Increased habitat structure does not always provide increased refuge from predation. Mar. Ecol. Prog. Ser. 2008, 361, 15–20. [Google Scholar] [CrossRef]
- Riisgård, H.U. Filter-feeding in the polychaete Nereis diversicolor: A review. Neth. J. Aquat. Ecol. 1994, 28, 453–458. [Google Scholar] [CrossRef]
- Pile, A.J.; Lipcius, R.N.; Van Montfrans, J.; Orth, R.J. Density dependent settler-recruit-juvenile relationships in blue crabs. Ecol. Monogr. 1996, 66, 277–300. [Google Scholar] [CrossRef]
- Martínez-Schönemann, A. Estimación de la Producción Primaria Neta de Epífitos en Praderas de Fanerógamas de la Bahía de Cádiz. Master’s Thesis, Univesity of Cádiz, Cádiz, Spain, 2014; 30p. [Google Scholar]
- Grignon-Dubois, M.; Rezzonico, B.; Alcoverro, T. Regional scale patterns in seagrass defences: Phenolic acid content in Zostera noltii. Estuar. Coast. Shelf Sci. 2012, 114, 18–22. [Google Scholar] [CrossRef]
- Manck, L.; Quintana, E.; Suarez, R.; Brun, F.G.; Hernández, I.; Ortega, M.J.; Zubia, E. Profiling of phenolic natural products in the seagrass Zostera noltei by UPLC-MS. Nat. Prod. Commun. 2017, 12, 687–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Variable | ZNNP | ZNAP | CNAP |
|---|---|---|---|
| Density (shoots m−2) | 8594 ± 1.198 | 3500 | 1700 |
| Above-ground biomass (g DW m−2) | 110.7 ± 35.6 | - | - |
| Leaf length (cm) | 19.1 ± 0.92 | 20 | 35 |
| Leaf width (cm) | 0.12 ± 0.02 | 0.5 | 0.6 |
| LAI (m2×m−2) | 3.27 ± 0.34 | 11.0 | 11.2 |
| Belowground biomass (g×DW×m−2) | 140.9 ± 22.8 | - | - |
| Belowground length (cm) | - | 30 | 30 |
| Belowground width (cm) | - | 0.32 | 0.44 |
| Volume occupied by belowground structures (%) | 15.7 ± 3.3 | 2.7 | 2.6 |
| Meadow area (m2) | - | ≈ 0.30 | ≈ 0.30 |
| BS | ZNAP | CNAP | ZNNP | |
|---|---|---|---|---|
| Number of species | 4 | 8 | 8 | 7 |
| Scrobicularia plana (%) | 82.6 ± 3.3 | 63.0 ± 2.4 | 61.7 ± 1.8 | 86.9 ± 2.6 |
| Hediste diversicolor (%) | 6.5 ± 0.8 | 33.0 ± 1.6 | 31.7 ± 2.1 | 7.1 ± 0.7 |
| Epiphyte (g DW×m−2) | - | 505 ± 29 | 1563 ± 111 | - |
| SOM (%) | 9.2 ± 0.5 | 8.3 ± 0.3 | 8.4 ± 0.3 | 9.2 ± 0.4 |
| Species and feeding behaviour | ||||
| Scrobicularia plana (SF/DF) | X | X | X | X |
| Cerastoderma edule (SF) | X | X | X | X |
| Venerupis rhomboides (SF) | X | |||
| Hediste diversicolor (O) | X | X | X | X |
| Marphysa sanguinea (DF) | X | X | X | |
| Bulla striata (C) | X | |||
| Venerupis sp. (SF) | X | |||
| Venerupis philippinarum (SF) | X | |||
| Solen marginatus (SF) | X | |||
| Diopatra neapolitana (C) | X | X | X | |
| Xantho pilipes (C) | X | |||
| Nephtys sp. (C/O) * | X | |||
| Crustacean (SF) # | X | X |
| Species-Site | ZNL | POM (<200 μm) | POM (>200 μm) | SOM | EPI |
|---|---|---|---|---|---|
| SP-BS | 0.37 (0.24–0.47) | 0.27 (0.07–0.46) | 0.13 (0.03–0.30) | 0.23 (0.05–0.47) | - |
| SP-ZNAP | 0.25 (0.07–0.38) | 0.53 (0.37–0.67) | 0.08 (0.002–0.20) | 0.07 (0.002–0.21) | 0.07 (0.007–0.16) |
| SP-CNAP | 0.20 (0.07–0.32) | 0.61 (0.49–0.73) | 0.05 (0.003–0.13) | 0.11 (0.02–0.23) | 0.05 (0.005–0.10) |
| SP-ZNNP | 0.35 (0.18–0.50) | 0.30 (0.04–0.55) | 0.11 (0.01–0.34) | 0.24 (0.03–0.46) | - |
| HD-BS | 0.64 (0.46–0.82) | 0.10 (0.004–0.31) | 0.13 (0.01–0.31) | 0.13 (0.01–0.37) | - |
| HD-ZNAP | 0.42 (0.23–0.59) | 0.15 (0.03–0.33) | 0.09 (0.02–0.24) | 0.08 (0.0–0.23) | 0.26 (0.11–0.41) |
| HD-CNAP | 0.44 (0.30–0.59) | 0.13 (0.02–0.30) | 0.08 (0.007–0.22) | 0.12 (0.02–0.24) | 0.22 (0.11–0.35) |
| HD-ZNNP | 0.73 (0.60–0.84) | 0.06 (0.001–0.23) | 0.09 (0.01–0.23) | 0.12 (0.01–0.26) | - |
| Group | SEAc | SEAc Overlap (%) |
|---|---|---|
| H. diversicolor | ||
| BS (1) | 0.76 | 1 vs. 2 (0.00) |
| 1 vs. 3 (0.00) | ||
| 1 vs. 4 (0.00) | ||
| ZNNP (2) | 0.19 | 2 vs. 3 (0.18) |
| 2 vs. 4 (1.76) | ||
| ZNAP (3) | 1.38 | 3 vs. 4 (62.4) |
| CNAP (4) | 0.99 | |
| S. plana | ||
| BS (1) | 0.02 | 1 vs. 2 (0.38) |
| 1 vs. 3 (0.25) | ||
| 1 vs. 4 (0.00) | ||
| ZNNP (2) | 0.96 | 2 vs. 3 (13.0) |
| 2 vs. 4 (9.37) | ||
| ZNAP (3) | 2.33 | 3 vs. 4 (17.3) |
| CNAP (4) | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brun, F.G.; Cobo-Díaz, J.F.; González-Ortiz, V.; Varela, J.L.; Pérez-Lloréns, J.L.; Vergara, J.J. Seagrass Patch Complexity Affects Macroinfaunal Community Structure in Intertidal Areas: An In Situ Experiment Using Seagrass Mimics. Diversity 2021, 13, 572. https://doi.org/10.3390/d13110572
Brun FG, Cobo-Díaz JF, González-Ortiz V, Varela JL, Pérez-Lloréns JL, Vergara JJ. Seagrass Patch Complexity Affects Macroinfaunal Community Structure in Intertidal Areas: An In Situ Experiment Using Seagrass Mimics. Diversity. 2021; 13(11):572. https://doi.org/10.3390/d13110572
Chicago/Turabian StyleBrun, Fernando G., José F. Cobo-Díaz, Vanessa González-Ortiz, José L. Varela, José Lucas Pérez-Lloréns, and Juan J. Vergara. 2021. "Seagrass Patch Complexity Affects Macroinfaunal Community Structure in Intertidal Areas: An In Situ Experiment Using Seagrass Mimics" Diversity 13, no. 11: 572. https://doi.org/10.3390/d13110572
APA StyleBrun, F. G., Cobo-Díaz, J. F., González-Ortiz, V., Varela, J. L., Pérez-Lloréns, J. L., & Vergara, J. J. (2021). Seagrass Patch Complexity Affects Macroinfaunal Community Structure in Intertidal Areas: An In Situ Experiment Using Seagrass Mimics. Diversity, 13(11), 572. https://doi.org/10.3390/d13110572