Alteriqipengyuania abyssalis sp. nov., a Novel Member of the Class Alphaproteobacteria Isolated from Sponge, and Emended Description of the Genus Alteriqipengyuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Cultivation and Maintenance of Bacteria
2.2. Morphological, Cultural and Physiological Characterization
2.3. 16S rRNA Gene Sequence and Phylogenetic Analysis
2.4. Chemotaxonomy
2.5. Genome Analysis
3. Results and Discussion
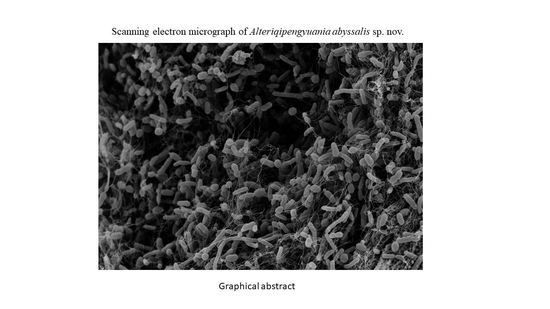
3.1. Morphological and Physiological Characteristics
3.2. 16S rRNA Gene-Based Phylogenetic Analysis
3.3. Chemotaxonomy
3.4. Genome Analysis
4. Emended Description of the Genus Alteriqipengyuania
5. Description of Alteriqipengyuania abyssalis sp. nov.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance; Government of the United Kingdom: London, UK, 2016.
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Tortorella, E.; Tedesco, P.; Esposito, F.P.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives. Mar. Drugs 2018, 16, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Sun, C.; Fang, C.; Oren, A.; Xu, X.W. Genomic-Based Taxonomic Classification of the Family Erythrobacteraceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 4470–4495. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Simidu, U. Erythrobacter Longus Gen. Nov., Sp. Nov., an Aerobic Bacterium Which Contains Bacteriochlorophyll A. Int. J. Syst. Bacteriol. 1982, 32, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.-T.; Park, S.; Lee, J.-S.; Yoon, J.-H. Erythrobacter lutimaris sp. nov., isolated from a tidal flat sediment. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 12, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C. LPSN—List of Prokaryotic Names with Standing in Nomenclature (Bacterio.Net), 20 Years On. Int. J. Syst. Evol. Microbiol. 2018, 68, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Lin, B.; Xu, L.; Li, G.; Wu, C.J.; Luo, L. Erythrobacter spongiae sp. nov., isolated from marine sponge. Int. J. Syst. Evol. Microbiol. 2019, 69, 1111–1116. [Google Scholar] [CrossRef]
- Denner, E.B.M.; Vybiral, D.; Koblízek, M.; Kämpfer, P.; Busse, H.-J.; Velimirov, B. Erythrobacter citreus sp. nov., a yellow-pigmented bacterium that lacks bacteriochlorophyll a, isolated from the western mediterranean sea. Int. J. Syst. Evol. Microbiol. 2002, 52, 1655–1661. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Zhang, H.; Chen, Y.; Li, Y.; Chen, Z.; Lai, Q.; Zhang, J.; Zheng, W.; Xu, H.; Zheng, T. Erythrobacter luteus sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2015, 65, 2472–2478. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Wu, Y.-H.; Sun, C.; Wang, H.; Cheng, H.; Meng, F.-X.; Wang, C.-S.; Xu, X.-W. Erythrobacter zhengii sp. nov., a bacterium isolated from deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2018, 69, 241–248. [Google Scholar] [CrossRef]
- Subhash, Y.; Tushar, L.; Sasikala, C.; Ramana, C.V.; Ch Ramana, C.V. Erythrobacter odishensis sp. nov. and Pontibacter odishensis sp. nov. isolated from dry soil of a solar saltern. Int. J. Syst. Evol. Microbiol. 2013, 63, 4524–4532. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Liu, Y.; Wang, N.; Xu, B.; Liu, K.; Shen, L.; Gu, Z.; Guo, B.; Zhou, Y.; Liu, H. Erythrobacter arachoides sp. nov., isolated from ice core. Int. J. Syst. Evol. Microbiol. 2017, 67, 4235–4239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Won, S.M.; Yoon, J.H. Erythrobacter marisflavi sp. nov., isolated from estuary water. Int. J. Syst. Evol. Microbiol. 2019, 69, 2696–2702. [Google Scholar] [CrossRef]
- Tonon, L.A.C.; Moreira, A.P.B.; Thompson, F. The Family Erythrobacteraceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9783642301, pp. 213–235. [Google Scholar] [CrossRef]
- Hosoya, S.; Arunpairojana, V.; Suwannachart, C.; Kanjana-Opas, A.; Yokota, A. Aureispira marina gen. nov., sp. nov., a gliding, arachidonic acid-containing bacterium isolated from the southern coastline of thailand. Int. J. Syst. Evol. Microbiol. 2006, 56, 2931–2935. [Google Scholar] [CrossRef] [Green Version]
- Landwehr, W.; Kämpfer, P.; Glaeser, S.P.; Rückert, C.; Kalinowski, J.; Blom, J.; Goesmann, A.; Mack, M.; Schumann, P.; Atasayar, E.; et al. Taxonomic Analyses of Members of the Streptomyces cinnabarinus Cluster, Description of Streptomyces cinnabarigriseus sp. nov. and Streptomyces davaonensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Correa, H.; Haltli, B.A.; Kerr, R.G. Fulvivirga aurantia sp. nov. and Xanthovirga aplysinae gen. nov., sp. nov., marine bacteria isolated from the sponge Aplysina fistularis, and Emended Description of the Genus Fulvivirga. Int. J. Syst. Evol. Microbiol. 2020, 70, 2766–2781. [Google Scholar] [CrossRef]
- Wu, H.X.; Lai, P.Y.; Lee, O.O.; Zhou, X.J.; Miao, L.; Wang, H.; Qian, P.Y. Erythrobacter Pelagi Sp. Nov., a Member of the Family Erythrobacteraceae isolated from the red sea. Int. J. Syst. Evol. Microbiol. 2012, 62, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K.I.; Garcia, R.O.; Gerth, K.; Irschik, H.; Müller, R. Sandaracinus Amylolyticus gen. nov., sp. nov., a starch-degrading soil myxobacterium, and description of Sandaracinaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete Genome Sequence of DSM 30083T, the Type Strain (U5/41T) of Escherichia coli, and a Proposal for Delineating Subspecies in Microbial Taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a Free Program for Phylogenetic Analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Pattengale, N.D.; Alipour, M.; Bininda-Emonds, O.R.P.; Moret, B.M.E.; Stamatakis, A. How Many Bootstrap Replicates Are Necessary? J. Comput. Biol. 2010, 17, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Minnikin, D.E.; O’Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An Integrated Procedure for the Extraction of Bacterial Isoprenoid Quinones and Polar Lipids. J. Microbiol. Methods 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Bóna-Lovász, J.; Bóna, A.; Ederer, M.; Sawodny, O.; Ghosh, R. A Rapid Method for the Extraction and Analysis of Carotenoids and Other Hydrophobic Substances Suitable for Systems Biology Studies with Photosynthetic Bacteria. Metabolites 2013, 3, 912–930. [Google Scholar] [CrossRef]
- Risdian, C.; Landwehr, W.; Rohde, M.; Schumann, P.; Hahnke, R.L.; Spröer, C.; Bunk, B.; Kämpfer, P.; Schupp, P.J.; Wink, J. Streptomyces bathyalis sp. nov., an actinobacterium isolated from the sponge in a deep sea. Antonie van Leeuwenhoek 2021, 114, 425–435. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An Algorithm That Identifies Contaminated Prokaryotic Genomes Using 16S RNA Gene Sequences. Int. J. Syst. Evol. Microbiol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Medema, M.H.; Blin, K.; Cimermancic, P.; De Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid Identification, Annotation and Analysis of Secondary Metabolite Biosynthesis Gene Clusters in Bacterial and Fungal Genome Sequences. Nucleic Acids Res. 2011, 39 (Suppl. 2), W339–W346. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. AntiSMASH 5.0: Updates to the Secondary Metabolite Genome Mining Pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M.; Spröer, C.; Klenk, H.P. When Should a DDH Experiment Be Mandatory in Microbial Taxonomy? Arch. Microbiol. 2013, 195, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Kosako, Y.; Yabuuchi, E.; Naka, T.; Fujiwara, N.; Kobayashi, K. Proposal of Sphingomonadaceae fam. nov., consisting of Sphingomonas Yabuuchi et Al. 1990, Erythrobacter Shiba and Shimidu 1982, Erythromicrobium Yurkov et Al. 1994, Porphyrobacter Fuerst et Al. 1993, Zymomonas Kluyver and van Niel 1936, and Sandaracinobac. Microbiol. Immunol. 2000, 44, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Rocha, J.; Martins, R.; Proença, D.N.; Morais, P.V.; Henriques, I.; Alves, A. Altererythrobacter halimionae sp. nov. and Altererythrobacter endophyticus sp. nov., Two Endophytes from the Salt Marsh Plant Halimione portulacoides. Int. J. Syst. Evol. Microbiol. 2017, 67, 3057–3062. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.I.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose Transporter 1, a Facilitated and High-Capacity Trehalose Transporter, Allows Exogenous Trehalose Uptake into Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, C.; Smorawinska, M.; Li, L.; Horikoshi, K. Comparison of the Gene Expression of Aspartate /2-D-Semialdehyde Dehydrogenase at Elevated Hydrostatic Pressure in Deep-Sea Bacteria 1. J. Biochem. 1997, 121, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Fatty Acids | 1 | 2 | 3 | 4 | 5 |
| Saturated | |||||
| C14:0 | 0.70 | 2.9 | - | - | - |
| C15:0 | 1.78 | - | - | 1.6 | 5.76 |
| C16:0 | 6.32 | 5.2 | 3.32 | 7.1 | 5.11 |
| C17:0 | 3.56 | 2.1 | 1.93 | 4.7 | 4.63 |
| C18:0 | - | - | - | TR | - |
| C19:0 | - | - | 1.63 | - | - |
| Branched | |||||
| C17:0a | 1.06 | - | 0.78 | - | 3.97 |
| Unsaturated | |||||
| C17:1ω6c | 22.88 | 29.0 | 17.76 | 13.8 | 34.69 |
| C18:1ω12t | 41.51 | - | - | - | - |
| C16:1ω7c | - | - | 6.75 | - | 3.04 |
| C16:1ω7t | - | - | 1.12 | - | - |
| C17:1ω8c | - | - | 1.69 | 1.5 | - |
| C18:1ω7c | - | - | - | - | 20.92 |
| Hydroxy | |||||
| C14:02-OH | 5.96 | 4.9 | 3.23 | 2.3 | 4.25 |
| C15:02-OH | - | 9.2 | 5.6 | TR | 9.9 |
| C16:02-OH | 0.96 | - | 3.77 | - | - |
| C18:1ω9t and/or C18:1ω7c. | - | 36.4 | 40.52 | - | - |
| Category | Subcategory | Subsystem | Role |
|---|---|---|---|
| Carbohydrate Metabolism | Di- and oligosaccharides | Trehalose Biosynthesis | Trehalose-6-phosphate phosphatase (EC 3.1.3.12) |
| Biosynthesis of amino acids ans derivates | Lysine, threonine, methionine and cystine. | Lysine biosynthesis DAP pathway | Aspartate-semialdehyde dehydrogenase (EC 1.2.1.11) |
| Motility and Chemotaxis | Prokaryotic flagellar motility | Flagellar motility | Flagellar biosynthesis protein FliR |
| Virulence, Disease and Defense | Resistance to antibiotics and toxic compounds | Multidrug Resistance Efflux Pumps | Multidrug and toxin extrusion (MATE) family |
| Resistance to antibiotics and toxic compounds | Cobalt-zinc-cadmium resistance | Cobalt-zinc-cadmium resistance protein CzcA |
| Reference Type Strains | 16S | ANI | dDDH |
|---|---|---|---|
| A. lutimaris S-5T | 98.58 | 82.18 | 19.90 |
| Q. pelagi UST081027-248T | 96.44 | 75.17 | 20.10 |
| Q. citreus RE35F/1T | 96.23 | 74.03 | 19.7 |
| Q. gaetbuli SW-161T | 95.77 | 74.50 | 19.5 |
| Q. flavus SW-46T | 95.63 | 74.25 | 20.30 |
| A. halimionae CPA5T | 94.78 | 74.51 | 20.30 |
| Characteristics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Motility | + | _ | _ | _ | + |
| Esterase (C 4) | w+ | _ | w+ | + | + |
| Esterase lipase (C 8) | w+ | _ | + | + | + |
| Cystine arylamidase | + | w+ | w+ | w+ | w+ |
| Trypsin | + | _ | w+ | + | w+ |
| α-chymotrypsin | + | + | + | _ | + |
| Naphthol-AS-BI-phosphohydrolase | + | + | w+ | + | + |
| N-acetyl-β-glucosaminidase | - | + | _ | _ | ND |
| lysine decarboxylase | w+ | ND | _ | _ | ND |
| Citrate utilization | w+ | ND | _ | _ | ND |
| Tryptophan deaminase | w+ | ND | + | + | ND |
| Gelatinase | + | _ | w+ | _ | ND |
| Nitrate reduction | _ | ND | _ | _ | _ |
| Esculin hydrolysis | + | ND | + | + | + |
| Mucic acid | _ | w+ | _ | + | ND |
| Acetoacetic acid | + | _ | + | w+ | ND |
| Polar lipids | DPG, PC, SGL, PG, L, PL1-3, GL1-3, PE, PME | * DPG, PG, PC, SGL, GL, L1-3 | ** DPG, PC, PE, PG,2L,1 unknown pigmented lipid P. | *** PE, PG, DPG, PC, SGL | **** PE, GL1-2, PL1-4. |
| Fatty acids | |||||
| C17:1ω6c | 22.88% | 29.0% | 34.69% | 17.76% | 7.66% |
| C14:02-OH | 5.96% | 4.9% | 4.25% | 3.23% | 5.89% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tareen, S.; Risdian, C.; Müsken, M.; Wink, J. Alteriqipengyuania abyssalis sp. nov., a Novel Member of the Class Alphaproteobacteria Isolated from Sponge, and Emended Description of the Genus Alteriqipengyuania. Diversity 2021, 13, 670. https://doi.org/10.3390/d13120670
Tareen S, Risdian C, Müsken M, Wink J. Alteriqipengyuania abyssalis sp. nov., a Novel Member of the Class Alphaproteobacteria Isolated from Sponge, and Emended Description of the Genus Alteriqipengyuania. Diversity. 2021; 13(12):670. https://doi.org/10.3390/d13120670
Chicago/Turabian StyleTareen, Sanaullah, Chandra Risdian, Mathias Müsken, and Joachim Wink. 2021. "Alteriqipengyuania abyssalis sp. nov., a Novel Member of the Class Alphaproteobacteria Isolated from Sponge, and Emended Description of the Genus Alteriqipengyuania" Diversity 13, no. 12: 670. https://doi.org/10.3390/d13120670
APA StyleTareen, S., Risdian, C., Müsken, M., & Wink, J. (2021). Alteriqipengyuania abyssalis sp. nov., a Novel Member of the Class Alphaproteobacteria Isolated from Sponge, and Emended Description of the Genus Alteriqipengyuania. Diversity, 13(12), 670. https://doi.org/10.3390/d13120670