Bivalve Diversity on the Continental Shelf and Deep Sea of the Perdido Fold Belt, Northwest Gulf of Mexico, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Data Analysis
3. Results
3.1. Taxonomic Composition
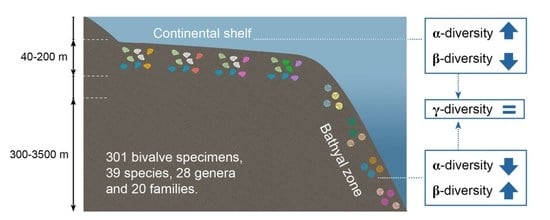
3.2. Patterns of α- and γ-Diversity
3.3. Patterns of β-Diversity
4. Discussion
4.1. Faunal Composition
4.2. Insights from Death Assemblages
4.3. Spatial Patterns of Species Diversity and Potential Drivers of Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Taxonomic List of the Identified Species
- Family Nuculidae Gray, 1824Nucula callicredemna Dall, 1890Material examined. Perdido 3, St. D4, 960 m, 1 empty shell (CMPY-001153).Overall geographic range. This species has recently been reported in deep waters in the eastern Gulf of Mexico, off Trinidad and Tobago, and Brazil, at depths of 1609–3731 m, benthic and infaunal [39].
- Nucula culebrensis E. A. Smith, 1885Material examined. Perdido 3, St.B3, 514 m, 1 empty shell (CMPY-001123) and St. C3, 508 m, 1 empty shell (CMPY-001199).Overall geographic range. This species has been reported in Texas, the Gulf of Mexico, and Puerto Rico, at depths of 417–1518 m [38].
- Nucula proxima Say, 1822Material examined. Perdido 3, St. B2, 48.8 m, 2 empty shells (CMPY-001122).Overall geographic range. The species occurring in Nova Scotia, Canada to Florida, and Texas, Bermuda, at depths of 0–124 m, benthic and infaunal [39].
- Family Nuculanidae H. Adams and A. Adams, 1858 (1854)Saccella acuta (Conrad, 1831)Material examined. Perdido 3, St. C1, 46.23 m, 1 empty shell (CMPY-001093), St. B1, 49.67 m, 1 empty shell (CMPY-001112) and 1 empty shell (CMPY-001119), St. C2, 98 m, 2 empty shells (CMPY-001253), 8 empty shells (CMPY-001138) and 1 empty shell (CMPY-001146), St. D1, 43 m, 1 empty shell (CMPY-001151), St. B2, 48.8 m, 2 empty shells (CMPY-001165), St. D2, 200 m, 3 empty shells (CMPY-001198) and 2 empty shells (CMPY-001200). Perdido 4, St. D1, 43.48 m, 1 empty shell (CMPY-002380), St. D2, 107.6 m, 1 empty shell (CMPY-002384) and 2 empty shells (CMPY-002438), St. F2, 93.38 m, 2 empty shells (CMPY-002440) and 3 empty shells (CMPY-002441), St. D6, 1660 m, 1 empty shell (CMPY-002439), St. C1, 48.23 m, 1 empty shell (CMPY-002378), 2 empty shells (CMPY-002434) and 1 empty shell (CMPY-002435), St. B1, 51.52 m, 1 empty shell (CMPY-002379), 1 empty shell (CMPY-002431) and 1 empty shell (CMPY-002432), St. B2, 96 m, 4 empty shells (CMPY-002433), St. C2, 104 m, 3 empty shells (CMPY-002436), 3 empty shells (CMPY-002437), and 1 empty shell (CMPY-002442).Overall geographic range. This species was found in Massachusetts to Campeche Bank, Mexico, West Indies, Brazil, and Cuba in the intervals at 0–274 m, benthic, estuarine, and infaunal [39].
- Saccella concentrica (Say, 1824)Figure 2E,FMaterial examined. Perdido 3, St. B3, 514 m, 2 empty shells (CMPY-001152), St. D1, 43 m, 3 empty shells (CMPY-001155), St. C1, 46.23 m, 1 empty shell (CMPY-001166). Perdido 4, St. F1, 48.7 m, 4 empty shells (CMPY-002355), St. D1, 43.48 m, 1 empty shell (CMPY-002443) and 8 empty shells (CMPY-002444), St. B1, 51.52, 1 empty shell (CMPY-002377).Overall geographic range. This species was found in west Florida to Texas, the northwest Gulf of Mexico offshore banks, and Brazil, at 0–90 m depth, benthic, infaunal, seagrass [39].
- Family Malletiidae H. Adams and A. Adams, 1858Katadesmia polita (Verrill and Bush, 1898)Material examined. Perdido 3, St. C1, 46.23 m, 1 empty shell (CMPY-001094). Perdido 4, St. F3, 375 m, 1 empty shell (CMPY-002387) and 1 empty shell (CMPY-002447), St. F4, 1378 m, 1 empty shell (CMPY-002454).Overall geographic range. Previously considered a synonym of Malletia bermudensis Hass, 1949 and Malletia polita Verrill and Bush, 1898.
- Family Neilonellidae Schileyko, 1989Neilonella sp.Material examined. Perdido 3, St. C5, 1371 m, 1 empty shell (CMPY-001254), St. D5, 1244 m, 1 empty shell (CMPY-001167). Perdido 4, St. B6, 1774 m, 2 empty shells (CMPY-002451).
- Family Tindariidae Verrill and Bush, 1897Tindaria amabilis (Dall, 1889)Material examined. Perdido 3, St. F3, 439 m, 2 empty shells (CMPY-001231), St. C7, 2644 m, 1 empty shell (CMPY-001140). Perdido 4, St. B5, 1543 m, 1 empty shell (CMPY-002367), St. B3, 438 m, 2 empty shells (CMPY-002357).Overall geographic range. The species occurring in the northwest Gulf of Mexico to the West Indies, Bermuda; Indian Ocean, at depths of 969–1829 m, benthic and infaunal [39].
- Tindaria sp.Material examined. Perdido 3, St. B5, 1500 m, 1 empty shell (CMPY-001193). Perdido 4, St. Chapo, 1315 m, 1 empty shell (CMPY-002453).
- aff. TindariaMaterial examined. Perdido 3, St. D4, 960 m, 2 empty shells (CMPY-001135).
- Family Yoldiidae Dall, 1908Orthoyoldia solenoides (Dall, 1881)Material examined. Perdido 3, St. F2, 86.4 m, 1 empty shell (CMPY-001159), St. C2, 98 m, 2 empty shell (CMPY-001091), St. C6, 2080 m, 2 empty shells (CMPY-001130). Perdido 4, St. F2, 93.38 m, 2 empty shells (CMPY-002352), 1 empty shell (CMPY-002373) and 1 empty shell (CMPY-002374), St. C2, 104 m, 2 empty shells (CMPY-002375).Live specimen. St. D1, 43 m, 1 live specimen (BCC-18082), Perdido 3.Overall geographic range. Previously considered a synonym of Nucula crosbyana Guppy, 1882 and Orthoyoldia solenoides Dall, 1881. The species occurs from Mississippi to Texas, at depths of 91–379 m, benthic and infaunal [39].
- Family Mytilidae Rafinesque, 1815Brachidontes sp.Material examined. Perdido 4, St. D1, 43.48 m, 1 empty shell (CMPY-002359), St. E7, 3053, 1 empty shells (CMPY-002452), St. F8, 3563 m, 1 empty shell (CMPY-002358).
- Family Arcidae Lamarck, 1809Anadara secticostata (Reeve, 1844)Material examined. Perdido 3, St. B4, 959.5 m, 1 empty shell (CMPY-001117), St. C2, 98 m, 1 empty shell (CMPY-001129), St. D2, 200 m, 3 empty shells (CMPY-001164).Overall geographic range. Previously considered a synonym of Anadara floridana (Conrad, 1869), Anomalocardia floridana Conrad, 1869, Arca lienosa Say, 1832 sensu Dall, 1886, Arca secticostata Reeve, 1844, and Scapharca crassissima Macsotay, 2001. The species occurs from North Carolina to Yucatan, Mexico, and Greater Antilles, at depths of 0–110 m, benthic, byssate, epibiotic, soft substrate (muds, sands, clays) [39].
- Bentharca asperula (Dall, 1881)Material examined. Perdido 3, St. D6, 1957 m, 1 empty shell (CMPY-001103). Perdido 4, St. C5, 1267 m, 1 empty shell (CMPY-002385).Overall geographic range. The species has been reported from Virginia to Florida and the northwest Gulf of Mexico, Cuba, and the Caribbean, at depths of 329–3475 m, benthic, byssate, and epibiotic [39].
- Bathyarca glomerula (Dall, 1881)Material examined. Perdido 4, St. Chapo, 1315 m, 1 empty shell (CMPY-002351), St. B5, 1543 m, 1 empty shell (CMPY-002376), St. F4, 1378 m, 1 empty shell (CMPY-002350).Live specimen. St. F8, 3563 m, 1 specimen (BCC-18315), Perdido 4.Overall geographic range. The species has been reported from North Carolina to southwest Florida, and the West Indies, at depths of 110–509 m, benthic and infaunal [39].
- Family Limopsidae Dall, 1895Limopsis cristata Jeffreys, 1876Material examined. Perdido 3, St. B4, 959.5 m, 3 empty shells (CMPY-001197).Overall geographic range. The species has been reported from Massachusetts to Dry Tortugas and off Texas, at depths of 55–1965 m, benthic, deep sea, and infaunal [39].
- Limopsis sulcata Verrill and Bush, 1898Material examined. Perdido 3, St. C4, 960 m, 1 empty shell (CMPY-001239), St. D4, 960 m, 4 empty shells (CMPY-001120), St. B6, 1794 m, 1 empty shell (CMPY-001121) and 3 empty shells (CMPY-001162). Perdido 4, St. C6, 1660 m, 1 empty shell (CMPY-002361), St. B6, 1774 m, 1 empty shell (CMPY-002356), St. B5, 1543 m, 2 empty shell (CMPY-002446).Overall geographic range. The species has been reported from Massachusetts to Campeche Bank, Mexico, and West Indies, at depths of 16–1401 m, benthic, deep sea, infaunal [39].
- Limopsis sp.Material examined. Perdido 3, St. C4, 960 m, 1 empty shell (CMPY-001118). Perdido 4, St. B3, 438 m, 2 empty shells (CMPY-002383).
- Family Pteriidae Gray, 1847 (1820)Isognomon bicolor (C.B. Adams, 1845)Figure 3J,KMaterial examined. Perdido 4, St. F2, 93.38 m, 2 empty shells (CMPY-002353), St. D2, 107.6 m, 1 empty shell (CMPY-002362).Overall geographic range. The species has been reported from Florida Keys and Dry Tortugas to Yucatan, Mexico, Cuba, West Indies, and Bermuda, at depths of 0–6 m, byssate, epibiotic, hard substrate, and inlet influenced [39].
- Family Pectinidae Rafinesque, 1815Hyalopecten strigillatus (Dall, 1889)Material examined. Perdido 3, St. B6, 1794 m, 1 empty shell (CMPY-001143). Perdido 4, St. B1, 51.52 m, 1 empty shell (CMPY-002354).Overall geographic range. Formerly in Cyclopecten (Mikkelsen and Bieler, 2008), the species has been reported off East Florida to northwest Gulf of Mexico, Cuba, at depths of 538–2160 m, benthic and epibiotic [39].
- Family Thyasiridae Dall, 1900 (1895)Thyasira trisinuata (d’Orbigny, 1853)Material examined. Perdido 3, St. B3, 514 m, 1 empty shell (CMPY-001137).Overall geographic range. The species has been reported in Nova Scotia, Canada to northwest Gulf of Mexico, West Indies; Alaska to California, at depths of 14–680 m, benthic; infaunal and soft substrate (muds, sands, clays) [39].
- Family Astartidae d’Orbigny, 1844 (1840)Astarte sp.Material examined. Perdido 3, St. C5, 1371 m, 2 empty shells (CMPY-001113), St. B6, 1794 m, 2 empty shells (CMPY-001127) and 2 empty shells (CMPY-001161), St. D5, 1244 m, 1 empty shell (CMPY-001196). Perdido 4, St. B5, 1543 m, 1 empty shell (CMPY-002448), St. Chapo, 1315 m, 2 empty shells (CMPY-002450), St. C5, 1267 m, 1 empty shell (CMPY-002360), St. B1, 51.52 m, 2 empty shells (CMPY-002370), St. F4, 1378 m, 2 empty shells (CMPY-002381) and 2 empty shells (CMPY-002449).
- Family Cardiidae Lamarck, 1809Microcardium sp.Material examined. Perdido 3, St. B2, 48.8 m, 1 empty shell (CMPY-001128).
- Family Veneridae Rafinesque, 1815Chione sp.Material examined. Perdido 4, St. C5, 1267 m, 1 empty shell (CMPY-002363).
- Family Tellinidae Blainville, 1814Macoploma extenuata (Dall, 1900)Material examined. Perdido 3, St. D1, 43 m, 2 empty shells (CMPY-001115).Live specimen. St. C4, 960 m, 1 specimen (BCC-18060), Perdido 3.Overall geographic range. Previously considered a synonym of Macoma extenuata Dall, 1900, the species has been reported from Florida Keys to Texas, at depths of 59–128 m, benthic; endemic to Gulf of Mexico and infaunal [39].
- Macoploma tageliformis (Dall, 1900)Material examined. Perdido 3, St. C1, 46.23 m, 3 empty shells (CMPY-001089).Overall geographic range. Previously considered a synonym of Macoma tageliformis Dall, 1900, the species has been reported in Florida Keys and Dry Tortugas to Quintana Roo, Mexico, Cuba, and Greater Antilles, Brazil, at depths of 0–49 m, benthic; estuarine and infaunal [39].
- Macoma sp.Material examined. Perdido 3, St. B1, 49.67 m, 1 empty shell (CMPY-001139).Eurytellina nitens (C.B. Adams, 1845)
- Material examined. Perdido 4, St. F2, 93.38 m, 1 empty shell (CMPY-002388).Overall geographic range. The species has been reported from North Carolina to Texas, Cuba, and Brazil, at depths of 0–120 m, benthic and infaunal [39].
- Family Myidae Lamarck, 1809Sphenia sp.Material examined. Perdido 4, St. F1, 48.7 m, 2 empty shells (CMPY-002382), St. D3, 379 m, 1 empty shell (CMPY-002371), St. B5, 1543 m, 1 empty shell (CMPY-002369), St. E7, 3053 m, 2 empty shells (CMPY-002368), St. F8, 3563 m, 1 empty shell (CMPY-002372), St. F5, 1905 m, 1 empty shell (CMPY-002365).
- Family Corbulidae Lamarck, 1818Caryocorbula contracta (Say, 1822)Material examined. Perdido 3, St. B1, 49.67 m, 11 empty shells (CMPY-001086), St. C1, 46.23 m, 4 empty shells (CMPY-001114), St. F1, 45.8 m, 4 empty shells (CMPY-001132), St. D1, 43 m, 8 empty shells (CMPY-001134), 22 empty shells (CMPY-001141) and 8 empty shells (CMPY-001131).Overall geographic range. The species has been reported from Massachusetts to Florida Keys, Texas, Cuba, West Indies, and Brazil, at depths of 0–14 m, benthic and infaunal [39].
- Caryocorbula dietziana (C.B. Adams, 1852)Material examined. Perdido 4, St. F1, 48.7 m, 1 empty shell (CMPY-002455), St. F2, 93.38 m, 6 empty shells (CMPY-002456), St. B1, 51.52 m, 2 empty shells (CMPY-002457), 1 empty shell (CMPY-002458) and 4 empty shells (CMPY-002460), St. C1, 48.23 m, 11 empty shells (CMPY-002459).Overall geographic range. The species has been reported in North Carolina, Gulf of Mexico, Cuba, Jamaica, and Brazil, at depths of 2–101 m, benthic and infaunal [39].
- Varicorbula limatula (Conrad, 1846)Material examined. Perdido 3, St. B2, 48.8 m, 4 empty shells (CMPY-001116), St. F2, 86.4 m, 3 empty shells (CMPY-001147), St. B1, 49.67 m, 3 empty shells (CMPY-001150), St. D2, 200 m, 4 empty shells (CMPY-001124). Perdido 4, St. D2, 107.6 m, 1 empty shell (CMPY-002461), St. D1, 43.48 m, 1 empty shell (CMPY-002462).Overall geographic range. Previously considered a synonym of Corbula disparilis d’Orbigny, 1853, Corbula limatula Conrad, 1846, and Varicorbula disparilis d’Orbigny, 1853, the species has been reported in North Carolina to Texas, Gulf of Mexico offshore banks, Cuba, West Indies, and Brazil, at depths of 0–549 m, benthic and infaunal [39].
- Corbula sp.Material examined. Perdido 4, St. C1, 48.23 m, 1 empty shell (CMPY-002366).
- Juliacorbula aequivalvis (Philippi, 1836)Material examined. Perdido 4, St. D1, 43.48 m, 8 empty shells (CMPY-002445).Overall geographic range. The species has been reported from Florida Straits to Florida Keys, North Gulf of Mexico, Cuba, Antilles, and Central America, at depths of 0–101 m, benthic and infaunal [39].
- Cardiomya sp.Material examined. Perdido 3, St. D2, 200 m, 1 empty shell (CMPY-001149).
- Family Cuspidariidae Dall, 1886Cuspidaria rostrata (Spengler, 1793)Material examined. Perdido 3, St. C2, 98 m, 1 empty shell (CMPY-001125) and 1 empty shell (CMPY-001148), St. D2, 200 m, 1 empty shell (CMPY-001163). Perdido 4, St. F2, 93.38 m, 1 empty shell (CMPY-002348), St. C2, 104 m, 1 empty shell (CMPY-002349).Overall geographic range. The species has been reported from the Arctic Ocean to Dry Tortugas, northwest Gulf of Mexico, and West Indies, at depths of 119–2926 m, benthic; deep sea and infaunal [39].
- Family Poromyidae Dall, 1886Poromya rostrata Rehder, 1943Material examined. Perdido 3, St. C2, 98 m, 1 empty shell (CMPY-001230).Overall geographic range. The species has been reported from North Carolina to Texas, West Indies, at depths of 0–200 m, benthic and infaunal [39].
- Poromyidae sp. 1Material examined. Perdido 4, St. B6, 1774 m, 2 empty shells (CMPY-002463).
- Poromyidae sp. 2Material examined. Perdido 4, St. C2, 104 m, 1 empty shell (CMPY-002464).
References
- Bray, R.A. Digenean parasites of deep-sea teleosts: A progress report. Int. J. Parasitol. Parasites Wildl. 2020, 12, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Chen, M.; Wicksten, M.K.; Rowe, G.T. Macrofauna bivalve diversity from the deep northern Gulf of Mexico. Ecol. Res. 2020, 35, 343–361. [Google Scholar] [CrossRef]
- Zezina, O.N. Biogeography of the bathyal zone. Adv. Mar. Biol. 1997, 32, 389–426. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R.; Smith, C.R. A riot of species in an environmental calm: The paradox of the species-rich deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 2002, 40, 311–342. [Google Scholar]
- Hessler, R.; Sanders, H.L. Faunal diversity in the deep-sea. Deep Sea Res. 1967, 14, 65–78. [Google Scholar] [CrossRef]
- Sanders, H.L.; Hessler, R.R. Ecology of the deep-sea benthos. Science 1969, 163, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Rex, M.A. Community Structure in the Deep-Sea Benthos. Annu. Rev. Ecol. Syst. 1981, 12, 331–353. [Google Scholar] [CrossRef]
- Olabarria, C. Patterns of bathymetric zonation of bivalves in the Porcupine Seabight and adjacent Abyssal plain, NE Atlantic. Deep-Sea Res. Part I 2005, 52, 15–31. [Google Scholar] [CrossRef]
- Levin, L.A.; Etter, R.J.; Rex, M.A.; Gooday, A.J.; Smith, C.R.; Pineda, J.; Stuart, C.T.; Hessler, R.R.; Pawson, D. Environmental influences on regional deep-sea species diversity. Annu. Rev. Ecol. Syst. 2001, 32, 51–93. [Google Scholar] [CrossRef] [Green Version]
- Hendrickx, M.E. Operaciones oceanográficas realizadas durante el proyecto TALUD en el Pacífico mexicano (1989–2009). In Biodiversidad y Comunidades del Talud Continental del Pacífico Mexicano; Instituto Nacional de Ecología, Secretaria del Medio Ambiente y Recursos Naturales (Semarnat): México City, Mexico, 2012; pp. 23–104. [Google Scholar]
- Aguilar, V. Análisis de vacíos y omisiones en conservación de la biodiversidad del mar profundo. In La Frontera Final: El Océano Profundo; Low Pfeng, A., Peters Recargno, E.M., Eds.; INECC: México City, Mexico, 2013; pp. 265–276. [Google Scholar]
- Rex, M.A.; Crame, J.A.; Stuart, C.T.; Clarke, A. Large-scale biogeographic patterns in marine mollusks: A confluence of history and productivity? Ecology 2005, 86, 2288–2297. [Google Scholar] [CrossRef]
- Rex, M.A.; McClain, C.R.; Johnson, N.A.; Etter, R.J.; Allen, J.A.; Bouchet, P.; Warén, A. A source-sink hypothesis for abyssal biodiversity. Am. Nat. 2005, 165, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Moretzsohn, F.; Tunnell, W., Jr.; Lyons, W.G.; Baqueiro, E.; Barrera, N.; Espinosa, E.; García, E.F.; Ortea, J.; Regueiro, M. Mollusca: Introduction. In Gulf of Mexico Origin, Waters, and Biota Volume 1, Biodiversity; Felder, D.L., Camp, D.K., Eds.; Texas A&M University Press: Corpus Christi, TX, USA, 2009; pp. 559–564. [Google Scholar]
- McClain, C.R.; Stegen, J.C.; Hurlbert, A.H. Dispersal, environmental niches and oceanic-scale turnover in deep-sea bivalves. Proc. R. Soc. B Biol. Sci. 2012, 279, 1993–2002. [Google Scholar] [CrossRef]
- Kamenev, G.M. Bivalve molluscs of the abyssal zone of the Sea of Okhotsk: Species composition, taxonomic remarks, and comparison with the abyssal fauna of the Pacific Ocean. Deep-Sea Res. Part II 2018, 154, 230–248. [Google Scholar] [CrossRef]
- Kamenev, G.M. Species composition and distribution of bivalves in bathyal and abyssal depths of the Sea of Japan. Deep-Sea Res. Part II 2013, 86–87, 124–139. [Google Scholar] [CrossRef]
- Kamenev, G.M. Composition and distribution of bivalves of the abyssal plain adjacent to the Kuril-Kamchatka Trench (Pacific Ocean). Deep-Sea Res. Part II 2015, 111, 188–197. [Google Scholar] [CrossRef]
- Rex, M.A.; Stuart, C.T.; Hessler, R.R.; Allen, J.A.; Sanders, H.L.; Wilson, G.D.F. Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature 1993, 365, 636–639. [Google Scholar] [CrossRef]
- Bürkli, A.; Wilson, A.B. Explaining high-diversity death assemblages: Undersampling of the living community, out-of-habitat transport, time-averaging of rare taxa, and local extinction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 466, 174–183. [Google Scholar] [CrossRef]
- Grill, B.; Zuschin, M. Modern shallow- to deep-water bivalve death assemblages in the Red Sea—Ecology and biogeography. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 168, 75–96. [Google Scholar] [CrossRef]
- Kidwell, S.M.; Tomasovych, A. Implications of Time-Averaged Death Assemblages for Ecology and Conservation Biology. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 539–563. [Google Scholar] [CrossRef] [Green Version]
- Kidwell, S.M. Ecological fidelity of open marine molluscan death assemblages: Effects of post-mortem transportation, shelf health, and taphonomic inertia. Lethaia 2008, 41, 199–217. [Google Scholar] [CrossRef]
- Kidwell, S.M. Discordance between living and death assemblages as evidence for anthropogenic ecological change. Proc. Natl. Acad. Sci. USA 2007, 104, 17701–17706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shantharam, A.K.; Baco, A.R. Biogeographic and bathymetric patterns of benthic molluscs in the Gulf of Mexico. Deep-Sea Res. Part I 2020, 155, 103167. [Google Scholar] [CrossRef]
- Gracia, A.; Valentich-Scott, P. New records of soft bottom bivalves (Mollusca) from the continental shelf and upper slope of the northern Pacific Ocean of Colombia. Mar. Biodivers. Rec. 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.; Taviani, M. Taxonomic, Ecological and Historical Considerations on the Deep-Water Benthic Mollusc Fauna of the Red Sea. In The Red Sea; Springer: Berlin/Heidelberg, Germany, 2015; pp. 511–529. [Google Scholar]
- Joye, S.B. The Gulf of Mexico ecosystem—Before, during and after the Deepwater Horizon oil well blowout. Deep-Sea Res. Part II 2016, 129, 1. [Google Scholar] [CrossRef]
- Salcedo, D.L.; Soto, L.A.; Estradas-Romero, A.; Botello, A.V. Interannual variability of soft-bottom macrobenthic communities of the NW Gulf of Mexico in relationship to the Deepwater Horizon oil spill. Mar. Pollut. Bull. 2017, 114, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Uritte, R.; Román, E.; Fkjres, H. El Cinturón Plegado Mexicano. Estructura y Potencial Petrolero. Boletín AMGP 2003, 50, 3–20. [Google Scholar]
- Gradmann, S.; Beaumont, C.; Albertz, M. Factors controlling the evolution of the Perdido Fold Belt, northwestern Gulf of Mexico, determined from numerical models. Tectonics 2009, 28. [Google Scholar] [CrossRef]
- Chen, M. Community Structure of Deep-Sea Bivalve Mollusks from the Northern Gulf of Mexico; Texas A&M University: Corpus Christi, TX, USA, 2004. [Google Scholar]
- Gustafson, R.G.; Turner, R.D.; Lutz, R.A.; Vrijenhoek, R.C. A new genus and five new species of mussels (Bivalvia, Mytilidae) from deep-sea sulfide/hydrocarbon seeps in the Gulf of Mexico. Malacologia 1998, 40, 63–112. [Google Scholar]
- McVeigh, D.M.; Eggleston, D.B.; Todd, A.C.; Young, C.M.; He, R. The influence of larval migration and dispersal depth on potential larval trajectories of a deep-sea bivalve. Deep-Sea Res. Part I 2017, 127, 57–64. [Google Scholar] [CrossRef]
- Vrijenhoek, R.C.; Schutz, S.J.; Gustafson, R.G.; Lutz, R.A. Cryptic species of deep-sea clams (Mollusca: Bivalvia: Vesicomyidae) from hydrothermal vent and cold-water seep environments. Deep-Sea Res. Part I 1994, 41, 1171–1189. [Google Scholar] [CrossRef]
- Wei, C.L.; Rowe, G.T.; Fain Hubbard, G.; Scheltema, A.H.; Wilson, G.D.F.; Petrescu, I.; Foster, J.M.; Wicksten, M.K.; Chen, M.; Davenport, R.; et al. Bathymetric zonation of deep-sea macrofauna in relation to export of surface phytoplankton production. Mar. Ecol. Prog. Ser. 2010, 399, 1–14. [Google Scholar] [CrossRef]
- Tunnell, J.W.; Andrews, J.; Barrera, N.C.; Moretzsohn, F. Encyclopedia of Texas Seashells: Identification, Ecology, Distribution, and History; Tunnell, J.W.J., Ed.; Texas A&M University Press: Corpus Christi, TX, USA, 2010; ISBN 9781603441414. [Google Scholar]
- Turgeon, D.D.; Lyons, W.G.; Mikkelsen, P.; Rosenberg, G.; Moretzsohn, F. Bivalvia (Mollusca) of the Gulf of Mexico. In Gulf of Mexico. Origin, Waters and Biota; Felder, D.L., Kamp, D.K., Eds.; Texas A&M University Press: College Station, TX, USA, 2009; pp. 711–744. [Google Scholar]
- Hernández-Ávila, I.; Pech, D.; Ocaña, F.A.; Árcega-Cabrera, F.; Enriquez, C. Shelf and deep-water benthic macrofauna assemblages from the western Gulf of Mexico: Temporal dynamics and environmental drivers. Mar. Environ. Res. 2021, 165, 105241. [Google Scholar] [CrossRef]
- Trudgill, B.D.; Rowan, M.G.; Fiduk, J.C.; Weimer, P.; Gale, P.E.; Korn, B.E.; Phair, R.L.; Gafford, W.T.; Roberts, G.R.; Dobbs, S.W. The Perdido Fold Belt, northwestern deep Gulf of Mexico; part 1, Structural geometry, evolution and regional implications. Am. Assoc. Pet. Geol. Bull. 1999, 83, 88–113. [Google Scholar]
- Baguley, J.G.; Montagna, P.A.; Hyde, L.J.; Kalke, R.D.; Rowe, G.T. Metazoan meiofauna abundance in relation to environmental variables in the northern Gulf of Mexico deep sea. Deep-Sea Res. Part I 2006, 53, 1344–1362. [Google Scholar] [CrossRef]
- Waller, T. Structural Analysis of the Perdido Fold Belt: Timing, Evolution, and Structural Style; Texas A&M University: College Station, TX, USA, 2007. [Google Scholar]
- Cisterna-Céliz, J.A.; Marcelino-Barros, M.; Herguera, J.C.; Rocha-Olivares, A. Metacommunity analysis of meiobenthos of deep-sea sediments from the Gulf of Mexico. Mar. Biodivers. 2019, 49, 1217–1231. [Google Scholar] [CrossRef]
- Díaz-Asencio, M.; Bartrina, V.F.; Herguera, J.C. Sediment accumulation patterns on the slopes and abyssal plain of the southern Gulf of Mexico. Deep-Sea Res. Part I 2019, 146, 11–23. [Google Scholar] [CrossRef]
- Dall, W.H. Reports on the results of dredging, under the supervision of Alexander Agassiz, in the Gulf of Mexico and in the Caribbean Sea (1877–78), by the United States Coast Survey Steamer “Blake”, Lieutenant-Commander C.D. Sigsbee, U.S.N., and Commander J.R. Bart. Bull. Mus. Comp. Zool. 1881, 9, 33–144. [Google Scholar]
- Abbott, R.T. American Seashells. The marine Mollusca of the Atlantic and Pacific Coast of North America; Van Nostrand Reinhold Company: New York, NY, USA, 1974; ISBN 9780442202286. [Google Scholar]
- Mikkelsen, P.M.; Bieler, R. Seashells of Southern Florida Living Marine Mollusks of the Florida Keys and Adjacent Regions; Princeton University Press: Princeton, NJ, USA, 2008; ISBN 13:978-0-691-11606-8. [Google Scholar]
- Redfern, C. Bahamian Seashells: 1161 Species from Abaco, Bahamas; Bahamianseashells.com, Inc.: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bouchet, P.; Rocroi, J.-P.; Bieler, R.; Carter, J.G.; Coan, E.V. Nomenclator of Bivalve Families with a Classification of Bivalve Families. Malacologia 2010, 52, 1–184. [Google Scholar] [CrossRef]
- WoRMS World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 20 June 2018).
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.R. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press Oxford: New York, NY, USA, 2011; pp. 39–54. [Google Scholar]
- Walther, B.A.; Moore, J.L. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Reese, G.C.; Wilson, K.R.; Flather, C.H. Performance of species richness estimators across assemblage types and survey parameters. Glob. Ecol. Biogeogr. 2014, 23, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: New York, NY, USA, 2002; ISBN 9780521811286. [Google Scholar]
- Anderson, M.J. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat. Sci. 2011, 58, 626–639. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Clarke, K.R.; Tweedley, J.R.; Valesini, F.J. Simple shade plots aid better long-term choices of data pre-treatment in multivariate assemblage studies. J. Mar. Biol. Assoc. UK 2014, 94, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013.
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.3-0; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Correa-Sandoval, A.; Rodríguez-Castro, J.H. Zoogeografía de los bivalvos marinos de la costa de Tamaulipas, México. Rev. Biol. Mar. Oceanogr. 2013, 48, 565–584. [Google Scholar] [CrossRef] [Green Version]
- García-Cubas, A. Ecología y Distribución de los Micromoluscos Recientes de la LAGUNA Madre, Tamaulipas, México; Universidad Nacional Autonoma de México, Instituto de Geología: Mexico City, Mexico, 1968. [Google Scholar]
- Secretaria de Marina. Fauna Malacológica del Área Costera de Tampico a Matamoros, Tamaulipas, México; Dirección General de Oceanografía: Ciudad de México, Mexico, 1980.
- Rice, W.H.; Kornicker, S. Mollusks of Alacran Reef, Campeche Bank, México. Publ. Inst. Mar. Sci. 1962, 8, 366–403. [Google Scholar]
- Washburn, T.; Rhodes, A.C.E.; Montagna, P.A. Benthic taxa as potential indicators of a deep-sea oil spill. Ecol. Indic. 2016, 71, 587–597. [Google Scholar] [CrossRef]
- Sabelli, B.; Taviani, M. The making of the Mediterranean Molluscan biodiversity. In The Mediterranean Sea: Its History and Present Challenges; Springer: Dordrecht, The Netherlands, 2014; Volume 9789400767, pp. 285–306. ISBN 9789400767041. [Google Scholar]
- Coan, E.V.; Valentich-Scott, P.; Sadeghian, P.S. Bivalve Seashells of Tropical West America. Marine Bivalve Mollusks from Baja California to Northern Peru. Museum of Natural History: Santa Barbara, CA, USA, 2012. [Google Scholar]
- Keen, A.M. Seashells of Tropical West America: Marine Mollusks from Baja California to Peru. Stanford University: Stanford, CA, USA, 1971. [Google Scholar]
- Rex, M.A. Deep-Sea Species Diversity: Decreased Gastropod Diversity at Abyssal Depths. Science 1973, 181, 1051–1053. [Google Scholar] [CrossRef]
- Escobar-Briones, E.; García-Villalobos, F.J. Distribution of total organic carbon and total nitrogen in deep-sea sediments from the southwestern Gulf of Mexico. Bol. Soc. Geol. Mex. 2009, 61, 73–86. [Google Scholar] [CrossRef]
- Brault, S.; Stuart, C.T.; Wagstaff, M.C.; McClain, C.R.; Allen, J.A.; Rex, M.A. Contrasting patterns of α- and Β-diversity in deep-sea bivalves of the eastern and western North Atlantic. Deep-Sea Res. Part II 2013, 92, 157–164. [Google Scholar] [CrossRef]
- McClain, C.R.; Lundsten, L.; Barry, J.; DeVogelaere, A. Assemblage structure, but not diversity or density, change with depth on a northeast Pacific seamount. Mar. Ecol. 2010, 31, 14–25. [Google Scholar] [CrossRef]
- Cartes, J.E.; Jaume, D.; Madurell, T. Local changes in the composition and community structure of suprabenthic peracarid crustaceans on the bathyal Mediterranean: Influence of environmental factors. Mar. Biol. 2003, 143, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Cartes, J.E.; Carrassón, M. Influence of trophic variables on the depth-range distributions and zonation rates of deep-sea megafauna: The case of the Western Mediterranean assemblages. Deep-Sea Res. Part I 2004, 51, 263–279. [Google Scholar] [CrossRef]
- McClain, C.R.; Rex, M.A. Toward a Conceptual Understanding of β-Diversity in the Deep-Sea Benthos. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 623–642. [Google Scholar] [CrossRef]







| Site | Date (d/m/yr) | Latitude (°N) | Longitude (°W) | Depth (m) | Zonation | Cruise |
|---|---|---|---|---|---|---|
| D1 | 9/06/2017 | 24°51.985′ | 97°17.431′ | 43 | Continental shelf | Perdido 3 |
| 1 | 8/06/2017 | 23°59.700′ | 97°32.855′ | 45.8 | Continental shelf | Perdido 3 |
| C1 | 10/06/2017 | 25°13.506′ | 97°03.913′ | 46 | Continental shelf | Perdido 3 |
| B2 | 10/06/2017 | 25°36.509′ | 96°34.057′ | 48.8 | Continental shelf | Perdido 3 |
| B1 | 10/06/2017 | 25°35.57′ | 96°52.179′ | 49.67 | Continental shelf | Perdido 3 |
| F2 | 8/06/2017 | 24°01.428′ | 97°18.686′ | 86.4 | Continental shelf | Perdido 3 |
| C2 | 10/06/2017 | 25°16.740′ | 96°42.041′ | 98 | Continental shelf | Perdido 3 |
| D2 | 9/06/2017 | 24°54.389′ | 96°51.808′ | 200 | Continental shelf | Perdido 3 |
| F3 | 8/06/2017 | 24°2.231′ | 97°5.234′ | 439 | Bathyal | Perdido 3 |
| C3 | 11/06/2017 | 25°16.755′ | 96°21.591′ | 508 | Bathyal | Perdido 3 |
| B3 | 11/06/2017 | 25°45.939′ | 96°14.808′ | 514 | Bathyal | Perdido 3 |
| B4 | 11/06/2017 | 25°35.795′ | 96°04.25′ | 959.5 | Bathyal | Perdido 3 |
| C4 | 14/06/2017 | 25°01.837′ | 96°18.620′ | 960 | Bathyal | Perdido 3 |
| D4 | 14/06/2017 | 24°46.555′ | 96°30.0015′ | 960 | Bathyal | Perdido 3 |
| D5 | 14/06/2017 | 24°54.6653′ | 96°08.8178′ | 1244 | Bathyal | Perdido 3 |
| C5 | 12/06/2017 | 25°16.420′ | 95°55.737′ | 1371 | Bathyal | Perdido 3 |
| B5 | 12/06/2017 | 25°34.969′ | 95°30.273′ | 1500 | Bathyal | Perdido 3 |
| B6 | 13/06/2017 | 25°46.2554′ | 95°24.832′ | 1794 | Bathyal | Perdido 3 |
| D6 | 13/06/2017 | 24°53.411′ | 95°56.540′ | 1957 | Bathyal | Perdido 3 |
| C6 | 12/06/2017 | 25°02.269′ | 95°47.748′ | 2080 | Bathyal | Perdido 3 |
| C7 | 13/06/2017 | 25°15.347′ | 95°21.281′ | 2644 | Bathyal | Perdido 3 |
| D1(1) | 20/09/2017 | 24°53.313′ | 97°17.421′ | 43.48 | Continental shelf | Perdido 4 |
| C1(1) | 24/09/2017 | 25°15.233′ | 97°2.974′ | 48.23 | Continental shelf | Perdido 4 |
| F1(1) | 19/09/2017 | 24°6.997′ | 97°32.212′ | 48.7 | Continental shelf | Perdido 4 |
| B1(1) | 24/09/2017 | 25°37.581 | 96°50.596′ | 51.52 | Continental shelf | Perdido 4 |
| F2(1) | 20/09/2017 | 24°1.479′ | 97°18.411′ | 93.38 | Continental shelf | Perdido 4 |
| B2(1) | 24/09/2017 | 25°38.112′ | 96°30.958′ | 96 | Continental shelf | Perdido 4 |
| C2(1) | 24/09/2017 | 25°15.195 | 96°42.419′ | 104 | Continental shelf | Perdido 4 |
| D2(1) | 20/09/2017 | 24°52.351′ | 96°53.716′ | 107.6 | Continental shelf | Perdido 4 |
| F3(1) | 20/09/2017 | 24°3.784′ | 97°5.182′ | 375 | Bathyal | Perdido 4 |
| D3 | 21/09/2017 | 24°53.778′ | 96°36.897′ | 379 | Bathyal | Perdido 4 |
| B3(1) | 24/09/2017 | 25°45.109′ | 96°16.363′ | 438 | Bathyal | Perdido 4 |
| C5(1) | 24/09/2017 | 25°11.221′ | 95°56.514′ | 1267 | Bathyal | Perdido 4 |
| Chapo | 23/09/2017 | 25°38.176′ | 95°34.583′ | 1315 | Bathyal | Perdido 4 |
| F4 | 28/09/2017 | 24°1.696′ | 96°40.524′ | 1378 | Bathyal | Perdido 4 |
| B5(1) | 23/09/2017 | 25°37.285′ | 95°29.292′ | 1543 | Bathyal | Perdido 4 |
| C6(1) | 22/09/2017 | 25°15.872′ | 95°39.099′ | 1660 | Bathyal | Perdido 4 |
| B6(1) | 23/09/2017 | 25°37.969′ | 95°24.863′ | 1774 | Bathyal | Perdido 4 |
| F5 | 27/09/2017 | 24°1.427′ | 96°26.205′ | 1905 | Bathyal | Perdido 4 |
| D6(1) | 22/09/2017 | 24°52.549′ | 95°54.934′ | 2130 | Bathyal | Perdido 4 |
| E7 | 25/09/2017 | 24°30.423′ | 95°41.441′ | 3053 | Bathyal | Perdido 4 |
| F8 | 26/09/2017 | 23°59.911′ | 95°13.687′ | 3563 | Bathyal | Perdido 4 |
| Family | Species (L/D) | Sites Perdido 3 | Sites Perdido 4 | Zonation |
|---|---|---|---|---|
| Nuculidae Gray 1824 | Nucula callicredemna Dall 1890 (D) | D4 | Bathyal | |
| Nucula culebrensis E. A. Smith 1885 (D) | B3, C3 | Bathyal | ||
| Nucula proxima Say 1822 (D) | B2 | Shelf | ||
| Nuculanidae H. Adams and A. Adams 1858 (1854) | Saccella acuta (Conrad 1831) (D) | C1, B1, C2, D1, B2, D2 | D1, D2, F2, D6, C1, B1, B2, C2 | Shelf and bathyal |
| Saccella concentrica (Say 1824) (D) | B3, D1, C1, F1, D1, B1 | Shelf and bathyal | ||
| Malletiidae H. Adams and A. Adams 1858 | Katadesmia polita (Verrill and Bush 1898) (D) | C1 | F3, F4 | Shelf and bathyal |
| Neilonellidae Schileyko 1989 | Neilonella sp. | C5, D5 | B6 | Bathyal |
| Tindariidae Verrill and Bush 1897 | Tindaria amabilis (Dall 1889) (D) | F3, C7 | B5, B3 | Bathyal |
| Tindaria sp. (D) | B5 | Chapo | Bathyal | |
| aff. Tindaria (D) | D4 | Bathyal | ||
| Yoldiidae Dall 1908 | Orthoyoldia solenoides (Dall 1881) (L/D) | F2, C2, C6, D1 | F2, C2 | Shelf and bathyal |
| Mytilidae Rafinesque 1815 | Brachidontes sp. (D) | D1, E7, F8 | Shelf and bathyal | |
| Arcidae Lamarck 1809 | Anadara secticostata (Reeve 1844) (D) | B4, C2, D2 | Shelf and bathyal | |
| Bentharca asperula (Dall 1881) (D) | D6 | C5 | Bathyal | |
| Bathyarca glomerula (Dall 1881) (L/D) | Chapo, B5, F4, F8 | Bathyal | ||
| Limopsidae Dall 1895 | Limopsis cristata Jeffreys 1876 (D) | B4 | Bathyal | |
| Limopsis sulcata Verrill and Bush 1898 (D) | C4, D4, B6 | C6, B6, B5 | Bathyal | |
| Limopsis sp. (D) | C4 | B3 | Bathyal | |
| Pteriidae Gray 1847 (1820) | Isognomon bicolor (C.B. Adams 1845) (D) | F2, D2 | Shelf | |
| Pectinidae Rafinesque 1815 | Hyalopecten strigillatus (Dall 1889) (D) | B6 | B1 | Shelf and bathyal |
| Thyasiridae Dall 1900 (1895) | Thyasira trisinuata (d’Orbigny 1853) (D) | B3 | Bathyal | |
| Astartidae d’Orbigny 1844 (1840) | Astarte sp. (D) | C5, B6, D5 | B5, Chapo, C5, B1, F4 | Shelf and bathyal |
| Cardiidae Lamarck 1809 | Microcardium sp. (D) | B2 | Shelf | |
| Veneridae Rafinesque 1815 | Chione sp. (D) | C5 | Bathyal | |
| Tellinidae Blainville 1814 | Macoploma extenuata (Dall 1900) (L/D) | D1, C4 | Shelf and bathyal | |
| Macoploma tageliformis (Dall 1900) (D) | C1 | Shelf | ||
| Macoma sp. (D) | B1 | Shelf | ||
| Eurytellina nitens (C.B.Adams 1845) (D) | F2 | Shelf | ||
| Myidae Lamarck 1809 | Sphenia sp. (D) | F1, D3, B5, E7, F8, F5 | Shelf and bathyal | |
| Corbulidae Lamarck 1818 | Caryocorbula contracta (Say 1822) (D) | B1, C1, F1, D1, | Shelf | |
| Caryocorbula dietziana (C.B. Adams 1852) (D) | F1, F2, B1, C1 | Shelf | ||
| Varicorbula limatula (Conrad 1846) (D) | B2, F2, B1, D2 | D2, D1 | Shelf | |
| Corbula sp. (D) | C1 | Shelf | ||
| Juliacorbula aequivalvis (Philippi 1836) (D) | D1 | Shelf | ||
| Cuspidariidae Dall 1886 | Cardiomya sp. (D) | D2 | Shelf | |
| Cuspidaria rostrata (Spengler 1793) (D) | C2, D2 | F2, C2 | Shelf | |
| Poromyidae Dall 1886 | Poromya rostrata Rehder 1943 (D) | C2 | Shelf | |
| Poromyidae sp. 1 (D) | B6 | Bathyal | ||
| Poromyidae sp. 2 (D) | C2 | Shelf |
| α-Diversity (±SD) | β-Diversity (MVD) | γ-Diversity | ||||
|---|---|---|---|---|---|---|
| Zone | n | Sobs | Chao2 (±SE) | Jack1 (±SE) | ||
| Shelf | 16 | 3.9 ± 1.5 | 50 | 25 | 77.7 ± 46.7 | 39.1 ± 4.5 |
| Bathyal | 26 | 2.0 ± 1.1 | 64 | 24 | 35.5 ± 8.8 | 35.5 ± 4.1 |
| A | ||||||
| Source | df | SS | MS | Pseudo-F | p | √CV |
| Cruise | 1 | 0.64 | 0.64 | 0.39 | 0.534 | 0 |
| Zonation | 1 | 35.72 | 35.72 | 21.59 | 0.001 | 1.31 |
| Cruise × Zonation | 1 | 0.17 | 0.17 | 0.10 | 0.771 | 0 |
| Residuals | 38 | 62.88 | 16.55 | 1.29 | ||
| Total | 41 | 99.62 | ||||
| B | ||||||
| Source | df | SS | MS | Pseudo-F | p | √CV |
| Cruise | 1 | 6774.4 | 6774.4 | 1.83 | 0.025 | 12 |
| Zonation | 1 | 25261 | 25261 | 6.83 | 0.001 | 33 |
| Cruise × Zonation | 1 | 4472.3 | 4472.3 | 1.21 | 0.240 | 9 |
| Residuals | 38 | 1.41 × 109 | 3699.6 | 61 | ||
| Total | 41 | 1.77 × 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Mozo, N.Y.; Vidal-Martínez, V.M.; Aguirre-Macedo, M.L.; Pech, D.; Guerra-Castro, E.; Simões, N. Bivalve Diversity on the Continental Shelf and Deep Sea of the Perdido Fold Belt, Northwest Gulf of Mexico, Mexico. Diversity 2021, 13, 166. https://doi.org/10.3390/d13040166
Suárez-Mozo NY, Vidal-Martínez VM, Aguirre-Macedo ML, Pech D, Guerra-Castro E, Simões N. Bivalve Diversity on the Continental Shelf and Deep Sea of the Perdido Fold Belt, Northwest Gulf of Mexico, Mexico. Diversity. 2021; 13(4):166. https://doi.org/10.3390/d13040166
Chicago/Turabian StyleSuárez-Mozo, Nancy Yolimar, Victor Manuel Vidal-Martínez, M. Leopoldina Aguirre-Macedo, Daniel Pech, Edlin Guerra-Castro, and Nuno Simões. 2021. "Bivalve Diversity on the Continental Shelf and Deep Sea of the Perdido Fold Belt, Northwest Gulf of Mexico, Mexico" Diversity 13, no. 4: 166. https://doi.org/10.3390/d13040166
APA StyleSuárez-Mozo, N. Y., Vidal-Martínez, V. M., Aguirre-Macedo, M. L., Pech, D., Guerra-Castro, E., & Simões, N. (2021). Bivalve Diversity on the Continental Shelf and Deep Sea of the Perdido Fold Belt, Northwest Gulf of Mexico, Mexico. Diversity, 13(4), 166. https://doi.org/10.3390/d13040166