The Diversity of Root-Associated Endophytic Fungi from Four Epiphytic Orchids in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Habitat and Sampling of the Terrestrial Orchids
2.2. Amplicon Sequencing and Analysis of RAF
2.3. Isolation and Identification of Culturable Endophytic Fungi
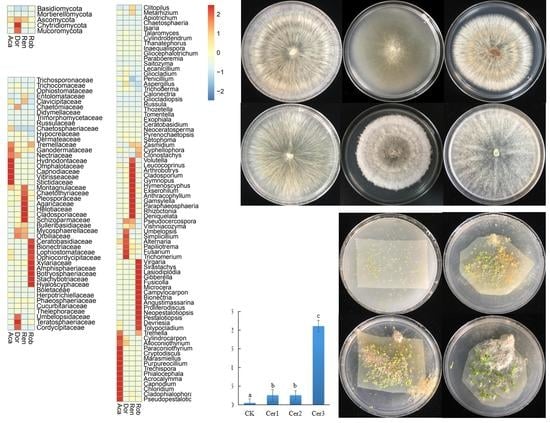
3. Results
3.1. Distribution of Endophytic Fungi in Root Cells
3.2. RAF Diversity
3.3. OMF Identities
3.4. Culturable Endophytic Fungi from the Orchid Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274. [Google Scholar] [CrossRef]
- Chen, X.Q.; Liu, Z.J.; Luo, Y.B.; Jin, X.H.; Ji, Z.H. A Field Guide to the Orchids of China; China Forestry Publishing House: Beijing, China, 2009; pp. 1–160. [Google Scholar]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Fay, M.F. Orchid conservation: Further links. Ann. Bot. 2016, 118, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Gebauer, G.; Preiss, K.; Gebauer, A.C. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytol. 2016, 211, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.-A. 12 Orchid Mycorrhizas: Molecular Ecology, Physiology, Evolution and Conservation Aspects. In Fungal Associations; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar]
- Ogura-Tsujita, Y.; Yokoyama, J.; Miyoshi, K.; Yukawa, T. Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am. J. Bot. 2012, 99, 1158–1176. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Song, L.; Zhao, Z.; Guo, S.; Xing, X. Mycorrhizal fungal community composition in seven orchid species inhabiting Song Mountain, Beijing, China. Sci. China Life Sci. 2019, 62, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Kohout, P.; Těšitelová, T.; Roy, M.; Vohník, M.; Jersáková, J. A diverse fungal community associated with Pseudorchis albida (Orchidaceae) roots. Fungal Ecol. 2013, 6, 50–64. [Google Scholar] [CrossRef]
- Suárez, J.P.; Weiß, M.; Abele, A.; Garnica, S.; Oberwinkler, F.; Kottke, I. Diverse tulasnelloid fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycol. Res. 2006, 110, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.P.; Weiss, M.; Oberwinkler, F.; Kottke, I. Epiphytic orchids in a mountain rain forest in southern Ecuador harbor groups of mycorrhiza-forming Tulasnellales and Sebacinales subgroup B (Basidiomycota). In Proceedings of the Second Scientific Conference on Andean Orchids; Universidad Técnica Particular de Loja: Loja, Ecuador, 2009; pp. 184–196. [Google Scholar]
- Addy, H.D.; Piercey, M.M.; Currah, R.S. Microfungal endophytes in roots. Can. J. Bot. 2005, 83, 1–13. [Google Scholar] [CrossRef]
- Bayman, P.; Otero, J.T. Microbial Endophytes of Orchid Roots; Schulz, B., Boyle, C., Sieber, T.N., Eds.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Yagame, T.; Orihara, T.; Selosse, M.; Yamato, M.; Iwase, K. Mixotrophy of Platanthera minor an orchid associated with ectomycorrhiza-forming Ceratobasidiaceae fungi. New Phytol. 2012, 193, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Cowden, C.C.; Shefferson, R.P. Diversity of root-associated fungi of mature Habenaria radiata and Epipactis thunbergii colonizing manmade wetlands in Hiroshima Prefecture, Japan. Mycoscience 2013, 54, 327–334. [Google Scholar] [CrossRef]
- Novotná, A.; Benítez, Á.; Herrera, P.; Cruz, D.; Filipczyková, E.; Suárez, J.P. High diversity of root-associated fungi isolated from three epiphytic orchids in southern Ecuador. Mycoscience 2018, 59, 24–32. [Google Scholar] [CrossRef]
- Jumpponen, A. Non-mycorrhizal root endophytes-aspects on their ecology. In Proceedings of the 7th International Mycological Congress, Oslo, Norway; Springer: Berlin, Germany, 2002. [Google Scholar]
- Otero, J.T.; Ackerman, J.D.; Bayman, P. Diversity and host specificity of endophytic Rhizoctonia -like fungi from tropical orchids. Am. J. Bot. 2002, 89, 1852–1858. [Google Scholar] [CrossRef]
- Ma, X.Y.; Kang, J.C.; Nontachaiyapoom, S.; Wen, T.; Hyde, K.D. Non-mycorrhizal endophytic fungi from orchids. Curr. Sci. 2015, 109, 72–87. [Google Scholar]
- Chen, J.; Meng, Z.X.; Xing, Y.M.; Guo, S.X. Isolation and Identification of Endophytic Fungi from Five Medicinal Plants Species of Orchidaceae. Chin. J. Pharm. 2017, 52, 267–271. [Google Scholar]
- Feng, X.X.; Chen, J.J.; Liu, F.; Hu, W.Z.; Lin, F.C.; Zhang, C.L. Diversity of non-mycorrhizal endophytic fungi from five epiphytic orchids from Xishuangbanna, China. Mycosystema 2019, 38, 1876–1885. [Google Scholar]
- Yuan, Z.-L.; Chen, Y.-C.; Yang, Y. Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): Estimation and characterization. World J. Microbiol. Biotechnol. 2009, 25, 295–303. [Google Scholar] [CrossRef]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Yang, J.-Z.; Liu, S.; Chen, C.-L.; Zhu, H.-Y.; Cao, J.-X. The colonization patterns of different fungi on roots of Cymbidium hybridum plantlets and their respective inoculation effects on growth and nutrient uptake of orchid plantlets. World J. Microbiol. Biotechnol. 2014, 30, 1993–2003. [Google Scholar] [CrossRef]
- Zhang, F.-S.; Lv, Y.-L.; Zhao, Y.; Guo, S.-X. Promoting role of an endophyte on the growth and contents of kinsenosides and flavonoids of Anoectochilus formosanus Hayata, a rare and threatened medicinal Orchidaceae plant. J. Zhejiang Univ. Sci. B 2013, 14, 785–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Zhang, K.; Cheng, S.; Nie, Q.; Zhou, S.-X.; Chen, Q.; Zhou, J.; Zhen, X.; Li, X.T.; Zhen, T.W.; et al. Fusarium oxysporum KB-3 from Bletilla striata: An orchid mycorrhizal fungus. Mycorrhiza 2019, 29, 531–540. [Google Scholar] [CrossRef]
- Vujanovic, V.; St-Arnaud, M.; Barabé, D.; Thibeault, G. Viability Testing of Orchid Seed and the Promotion of Colouration and Germination. Ann. Bot. 2000, 86, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.R.; Stewart, S.L.; Dutra, D.; Kane, M.E.; Richardson, L. Asymbiotic and symbiotic seed germination of Eulophia alta (Orchidaceae)—Preliminary evidence for the symbiotic culture advantage. Plant Cell Tissue Organ Cult. 2007, 90, 313–323. [Google Scholar] [CrossRef]
- Ovando, I.; Damon, A.; Bello, R.; Ambrosio, D.; Albores, V.; Adriano, L.; Salvador, M. Isolation of endophytic fungi and their potential for the tropical epiphytic orchids Cattleya skinneri, C. aurantiaca and Brassavola nodosa. Asian J. Plant Sci. 2005, 4, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Ding, S.Y.; Lai, Q.X.; Xiao, H.X.; Li, H.W. Investigation report on orchid germplasm resources in Hainan Island. Chin. J. Trop. Crops 1991, 12, 105–111. [Google Scholar]
- Tsi, Z.H.; Chen, X.Q.; Ding, S.Y. Critical and additional notes on orchids of Hainan, China. ACTA Phytotaxon. Sin. 1995, 33, 576–591. [Google Scholar]
- Shi, G.Z.; Zhou, T.F.; Yin, G.T. Distribution and conservation strategies for wild orchid resources in Jianfengling, Hailan Island. J. Fujian For. Sci. Technol. 2008, 35, 203–207. [Google Scholar]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Magoč, M.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.T.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Inviron. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolde, R.; Kolde, M.R. Package Pheatmap 1.0.12. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 4 January 2019).
- Toju, H.; Sato, H.; Yamamoto, S.; Kadowaki, K.; Tanabe, A.S.; Yazawa, S.; Nishimura, O.; Agata, K. How are plant and fungal communities linked to each other in belowground ecosystems? A massively parallel pyrosequencing analysis of the association specificity of root-associated fungi and their host plants. Ecol. Evol. 2013, 3, 3112–3124. [Google Scholar] [CrossRef]
- Almario, J.; Jeena, G.; Wunder, J.; Langen, G.; Zuccaro, A.; Coupland, G.; Bucher, M. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc. Natl. Acad. Sci. USA 2017, 114, E9403–E9412. [Google Scholar] [CrossRef] [Green Version]
- Toghueo, R.M.K.; Boyom, F.F. Endophytic Fungi from Terminalia Species: A Comprehensive Review. J. Fungi 2019, 5, 43. [Google Scholar] [CrossRef]
- Huang, W.Y.; Cai, Y.Z.; Hyde, K.D.; Corke, H.; Sun, M. Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Diver. 2008, 33, 61–75. [Google Scholar]
- Yang, S.Q.; Zhang, Q.; Song, X.Q.; Wang, J.; Li, Y.D.; Xu, H.; Guo, S.Y.; Ding, Q. Structural features of root-associated fungus-plant interaction networks in the tropical montane rain forest of Jianfengling, China (in Chinese). Biodiver. Sci. 2019, 27, 314–326. [Google Scholar]
- Chai, X.L.; Song, X.Q.; Zhu, J. Diversity of Endophytic Fungi Isolated from Dendrobium sinense with Different Culture Media and Their Antimicrobial Activities. Chin. J. Trop. Crops 2018, 39, 137–144. [Google Scholar]
- Li, J.J. Epiphytic characteristic and the diversity of root endophytic fungi of Oxystophyllum changjiangense in Bawangling, Hainan Island. Ph.D. Thesis, Hainan University, Hainan, China, February 2016. [Google Scholar]
- Deng, W.X.; Zhao, M.L.; Li, Y.M.; Wang, Z.L.; Zhang, K.; Zhan, F.D. Diversity of endophytic fungi associated with Bletilla striata roots (in Chinese). Mycosystema 2019, 38, 1907–1917. [Google Scholar]
- Ke, H.L.; Song, X.Q.; Tan, Z.Q.; Liu, H.X.; Luo, Y.B. Endophytic fungi diversity in root of Doritis pulcherrima (Orchidaceae). Biodiv. Sci. 2007, 15, 456–462. [Google Scholar]
- Cohen, S.D. Host Selectivity and Genetic Variation of Discula umbrinella Isolates from Two Oak Species: Analyses of Intergenic Spacer Region Sequences of Ribosomal DNA. Microb. Ecol. 2006, 52, 463–469. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Ferraro, V.; Burruano, S.; Lopez-Llorca, L.V. Fungal Assemblages Associated with Roots of Halophytic and Non-halophytic Plant Species Vary Differentially Along a Salinity Gradient. Microb. Ecol. 2012, 64, 668–679. [Google Scholar] [CrossRef] [Green Version]
- Girlanda, M.; Segreto, R.; Cafasso, D.; Liebel, H.T.; Rodda, M.; Ercole, E.; Cozzolino, S.; Gebauer, G.; Perotto, S. Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. Am. J. Bot. 2011, 98, 1148–1163. [Google Scholar] [CrossRef] [Green Version]
- Jacquemyn, H.; Honnay, O.; Cammue, B.P.A.; Brys, R.; Lievens, B. Low specificity and nested subset structure characterize mycorrhizal associations in five closely related species of the genus Orchis. Mol. Ecol. 2010, 19, 4086–4095. [Google Scholar] [CrossRef]
- Waud, M.; Busschaert, P.; Ruyters, S.; Jacquemyn, H.; Lievens, B. Impact of primer choice on characterization of orchid mycorrhizal communities using 454 pyrosequencing. Mol. Ecol. Resour. 2014, 14, 679–699. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, L.; Girlanda, M.; Kull, T.; Perini, C.; Perotto, S. Analysis of fungal diversity in Orchis tridentata Scopoli. Open Life Sci. 2012, 7, 850–857. [Google Scholar] [CrossRef]
- Pereira, O.L.; Kasuya, M.C.M.; Borges, A.C.; De Araújo, E.F. Morphological and molecular characterization of mycorrhizal fungi isolated from neotropical orchids in Brazil. Can. J. Bot. 2005, 83, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Otero, J.T.; Flanagan, N.S.; Herre, E.A.; Ackerman, J.D.; Bayman, P. Wide spread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae). Am. J. Bot. 2007, 94, 1944–1950. [Google Scholar] [CrossRef] [Green Version]
- Otero, J.T.; Bayman, P.; Ackerman, J.D. Variation in mycorrhizal performance in the epiphytic orchid Tolumnia variegata in vitro: The potential for natural selection. Evol. Ecol. 2005, 19, 29–43. [Google Scholar] [CrossRef]
- Carlsward, B.S.; Whitten, W.M.; Williams, N.H.; Bytebier, B. Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leaflessness. Am. J. Bot. 2006, 93, 770–786. [Google Scholar] [CrossRef] [Green Version]
- Hoang, N.H.; Kane, M.E.; Radcliffe, E.N.; Zettler, L.W.; Richardson, L.W. Comparative seed germination and seedling development of the ghost orchid, Dendrophylax lindenii (Orchidaceae), and molecular identification of its mycorrhizal fungus from South Florida. Ann. Bot. 2016, 119, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Richardson, K.; Currah, R.; Hambleton, S. Basidiomycetous endophytes from the roots of neotropical epiphytic Orchidaceae. Lindleyana 1993, 8, 127–137. [Google Scholar]
- Cameron, D.D.; Leake, J.R.; Read, D.J. Mutualistic mycorrhiza in orchids: Evidence from plant–fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol. 2006, 171, 405–416. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Cowden, C.C.; McCormick, M.K.; Yukawa, T.; Ogura-Tsujita, Y.; Hashimoto, T. Evolution of host breadth in broad interactions: Mycorrhizal specificity in East Asian and North American rattlesnake plantains (Goodyera spp.) and their fungal hosts. Mol. Ecol. 2010, 19, 3008–3017. [Google Scholar] [CrossRef]
- Suetsugu, K.; Yamato, M.; Matsubayashi, J.; Tayasu, I. Comparative study of nutritional mode and mycorrhizal fungi in green and albino variants of Goodyera velutina, an orchid mainly utilizing saprotrophic rhizoctonia. Mol. Ecol. 2019, 28, 4290–4299. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Freudenstein, J.V.; Rasmussen, F.N.; Rasmussen, H.N. Molecular identification of mycorrhizal fungi in Neuwiedia veratrifolia (Orchidaceae). Mol. Phylogenetics Evol. 2004, 33, 251–258. [Google Scholar] [CrossRef]
- Pecoraro, L.; Huang, L.Q.; Caruso, T.; Perotto, S.; Girlanda, M.; Cai, L.; Liu, Z.J. Fungal diversity and specificity in Cephalanthera damasonium and C. longifolia (Orchidaceae) mycorrhizas. J. Syst. Evol. 2017, 55, 69–158. [Google Scholar] [CrossRef] [Green Version]
- Pecoraro, L.; Caruso, T.; Cai, L.; Gupta, V.K.; Liu, Z.-J. Fungal networks and orchid distribution: New insights from above- and below-ground analyses of fungal communities. IMA Fungus 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán-López, M.; Caroca-Cáceres, R.; Jahreis, K.; Narváez-Vera, M.; Ansaloni, R.; Cazar, M. The micorryzal fungi Ceratobasidium sp. and Sebacina vermifera promote seed germination and seedling development of the terrestrial orchid Epidendrum secundum Jacq. S. Afr. J. Bot. 2019, 125, 54–61. [Google Scholar] [CrossRef]
- Yang, Q.Y.; He, C.F.; Liang, L.X.; Wang, T.; Liu, L. Effects of mycorrhizal fungi on the growth of three species of orchid seedlings. J. Nuclear Agric. Sci. 2019, 33, 0687–0695. [Google Scholar]





| Sample ID | Total Tags | Unique Tags | Taxon Tags | OTUs | Total OTUs |
|---|---|---|---|---|---|
| Dor-1 | 69,454 | 18,165 | 62,629 | 642 | 1060 |
| Dor-2 | 72,288 | 17,112 | 58,326 | 580 | |
| Dor-3 | 71,562 | 17,193 | 59,545 | 561 | |
| Ren-1 | 59,213 | 14,312 | 48,183 | 401 | 942 |
| Ren-2 | 56,112 | 14,048 | 26,360 | 486 | |
| Ren-3 | 61,040 | 17,035 | 47,652 | 444 | |
| Aca-1 | 66,909 | 16,981 | 57,335 | 544 | 1256 |
| Aca-2 | 70,557 | 21,830 | 57,532 | 858 | |
| Aca-3 | 58,702 | 17,499 | 31,089 | 538 | |
| Rob-1 | 73,556 | 22,602 | 64,663 | 1014 | 1500 |
| Rob-2 | 63,368 | 20,344 | 59,798 | 1011 | |
| Rob-3 | 70,926 | 22,675 | 49,842 | 902 |
| OTU | Aca | Dor | Ren | Rob | Family |
|---|---|---|---|---|---|
| Otu000006 | 221 | 50 | 14 | 29,765 | Ceratobasidiaceae |
| Otu000034 | 4 | 8 | 0 | 0 | Russulaceae |
| Otu000036 | 0 | 9 | 0 | 0 | Ceratobasidiaceae |
| Otu000263 | 0 | 61 | 528 | 5 | Ceratobasidiaceae |
| Otu000347 | 11 | 0 | 0 | 382 | Ceratobasidiaceae |
| Otu000506 | 0 | 0 | 224 | 0 | Ceratobasidiaceae |
| Otu000586 | 2 | 0 | 0 | 174 | Ceratobasidiaceae |
| Otu000700 | 0 | 0 | 0 | 2 | Thelephoraceae |
| Otu000812 | 0 | 7 | 91 | 0 | Ceratobasidiaceae |
| Otu001522 | 0 | 0 | 0 | 1 | Thelephoraceae |
| Otu001615 | 0 | 0 | 28 | 0 | Ceratobasidiaceae |
| Otu003239 | 0 | 0 | 8 | 0 | Ceratobasidiaceae |
| Otu003259 | 0 | 0 | 7 | 0 | Thelephoraceae |
| Otu003568 | 6 | 0 | 0 | 0 | Thelephoraceae |
| Otu003935 | 1 | 2 | 2 | 0 | Ceratobasidiaceae |
| Otu004007 | 5 | 0 | 0 | 0 | Thelephoraceae |
| Otu004486 | 0 | 0 | 0 | 4 | Ceratobasidiaceae |
| Otu004340 | 0 | 0 | 2 | 0 | Ceratobasidiaceae |
| Otu004661 | 0 | 4 | 0 | 0 | Ceratobasidiaceae |
| Otu004729 | 0 | 0 | 0 | 3 | Thelephoraceae |
| Otu004970 | 3 | 0 | 0 | 0 | Sebacinaceae |
| Otu005044 | 3 | 0 | 0 | 0 | Sebacinaceae |
| Otu005345 | 3 | 0 | 0 | 0 | Thelephoraceae |
| Otu005532 | 2 | 0 | 0 | 0 | Thelephoraceae |
| Otu005951 | 0 | 0 | 0 | 2 | Thelephoraceae |
| Otu006537 | 0 | 2 | 0 | 0 | Russulaceae |
| Otu006568 | 2 | 0 | 0 | 0 | Thelephoraceae |
| Otu006636 | 0 | 0 | 2 | 0 | Ceratobasidiaceae |
| OTU | OTU in This Study | Source Species | Closest Matches in GenBank | Identity (%) |
|---|---|---|---|---|
| Cer1 | Otu004661 | D. pulcherrima | MG654436.1 Ceratobasidium sp. isolate 85, Triticum aestivum root associated fungus, Azerbaijan | 99.66 |
| Cer2 | Otu004340 | R. coccinea | DQ102402.1 Ceratobasidium sp. AG-G isolate Str14, Fragaria x ananassa associated fungus, Israel | 98.21 |
| Cer3 | Otu000036 | R. coccinea | EF536969.1 Ceratobasidium sp. FPUB 168, Dactylorhiza hatagirea (Orchidaceae) root associated fungus, India | 100 |
| Cer4 | Otu004661 | D. pulcherrima | MG654436.1 Ceratobasidium sp. isolate 85, Triticum aestivum root associated fungus, Azerbaijan | 100 |
| Cer5 | Otu000006 | R. succisa | JQ713569.1 Ceratobasidium sp. isolate M13, Rhynchostylis retusa (Orchidaceae) root associated fungus, China | 99.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chi, M.; Guo, L.; Liu, D.; Yang, Y.; Zhang, Y. The Diversity of Root-Associated Endophytic Fungi from Four Epiphytic Orchids in China. Diversity 2021, 13, 197. https://doi.org/10.3390/d13050197
Wang T, Chi M, Guo L, Liu D, Yang Y, Zhang Y. The Diversity of Root-Associated Endophytic Fungi from Four Epiphytic Orchids in China. Diversity. 2021; 13(5):197. https://doi.org/10.3390/d13050197
Chicago/Turabian StyleWang, Tao, Miao Chi, Ling Guo, Donghuan Liu, Yu Yang, and Yu Zhang. 2021. "The Diversity of Root-Associated Endophytic Fungi from Four Epiphytic Orchids in China" Diversity 13, no. 5: 197. https://doi.org/10.3390/d13050197
APA StyleWang, T., Chi, M., Guo, L., Liu, D., Yang, Y., & Zhang, Y. (2021). The Diversity of Root-Associated Endophytic Fungi from Four Epiphytic Orchids in China. Diversity, 13(5), 197. https://doi.org/10.3390/d13050197