Does Hyperoxia Restrict Pyrenean Rock Lizards Iberolacerta bonnali to High Elevations?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
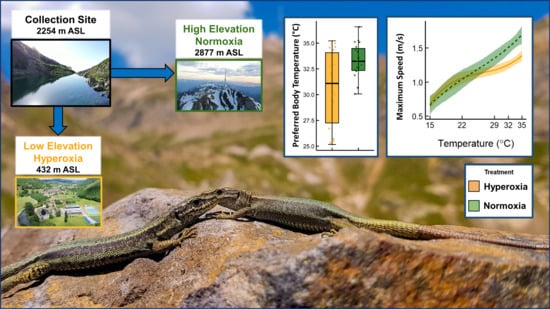
2.2. Experimental Design
2.3. Thermal Preferences
2.4. Performance Measurements
2.5. Ethics Statement
2.6. Statistical Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayre, R.; Frye, C.; Karagulle, D.; Krauer, J.; Breyer, S.; Aniello, P.; Wright, D.J.; Payne, D.; Adler, C.; Warner, H.; et al. A New High-Resolution Map of World Mountains and an Online Tool for Visualizing and Comparing Characterizations of Global Mountain Distributions. Mt. Res. Dev. 2018, 38, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Hoorn, C.; Mosbrugger, V.; Mulch, A.; Antonelli, A. Biodiversity from mountain building. Nat. Geosci. 2013, 6, 154. [Google Scholar] [CrossRef]
- Shen, C.; Shi, Y.; Fan, K.; He, J.-S.; Adams, J.M.; Ge, Y.; Chu, H. Soil pH dominates elevational diversity pattern for bacteria in high elevation alkaline soils on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, A.J.; Freeman, K.R.; McCormick, K.F.; Lynch, R.C.; Lozupone, C.; Knight, R.; Schmidt, S.K. Biogeography and habitat modelling of high-alpine bacteria. Nat. Commun. 2010, 1, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birrell, J.H.; Shah, A.A.; Hotaling, S.; Giersch, J.J.; Williamson, C.E.; Jacobsen, D.; Woods, H.A. Insects in high-elevation streams: Life in extreme environments imperiled by climate change. Glob. Chang. Biol. 2020, 26, 6667–6684. [Google Scholar] [CrossRef]
- Mani, M.S. Ecology and Biogeography of High Altitude Insects; Springer: Berlin/Heidelberg, Germany, 2013; Volume 4. [Google Scholar]
- Thaler, K. The diversity of high altitude arachnids (Araneae, Opiliones, Pseudoscorpiones) in the Alps. In Alpine Biodiversity in Europe; Springer: Berlin/Heidelberg, Germany, 2003; pp. 281–296. [Google Scholar]
- Baur, B.; Meier, T.; Baur, A.; Schmera, D. Terrestrial gastropod diversity in an alpine region: Disentangling effects of elevation, area, geometric constraints, habitat type and land-use intensity. Ecography 2014, 37, 390–401. [Google Scholar] [CrossRef]
- Ports, M. Habitat affinities and distributions of land gastropods from the Ruby Mountains and East Humboldt Range of northeastern Nevada. Veliger 1996, 39, 335–341. [Google Scholar]
- Atkinson, C.L.; Alexiades, A.V.; MacNeill, K.L.; Encalada, A.C.; Thomas, S.A.; Flecker, A.S. Nutrient recycling by insect and fish communities in high-elevation tropical streams. Hydrobiologia 2019, 838, 13–28. [Google Scholar] [CrossRef]
- Tong, C.; Fei, T.; Zhang, C.; Zhao, K. Comprehensive transcriptomic analysis of Tibetan Schizothoracinae fish Gymnocypris przewalskii reveals how it adapts to a high altitude aquatic life. BMC Evol. Biol. 2017, 17, 74. [Google Scholar] [CrossRef] [Green Version]
- Cordier, J.M.; Lescano, J.N.; Ríos, N.E.; Leynaud, G.C.; Nori, J. Climate change threatens micro-endemic amphibians of an important South American high-altitude center of endemism. Amphib. Reptil. 2020, 41, 233–243. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhang, Y.; Lee, W.-H.; Zhang, Y. Rich diversity and potency of skin antioxidant peptides revealed a novel molecular basis for high-altitude adaptation of amphibians. Sci. Rep. 2016, 6, 19866. [Google Scholar] [CrossRef]
- Storz, J.F. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 2007, 88, 24–31. [Google Scholar] [CrossRef]
- Badgley, C. Tectonics, topography, and mammalian diversity. Ecography 2010, 33, 220–231. [Google Scholar] [CrossRef]
- Fjeldså, J.; Bowie, R.; Rahbek, C. The role of mountain ranges in the diversification of birds. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Cerdeña, J.; Farfán, J.; Quiroz, A.J. A high mountain lizard from Peru: The world’s highest-altitude reptile. Herpetozoa 2021, 34, 61. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of reptile elevational diversity. Glob. Ecol. Biogeogr. 2010, 19, 541–553. [Google Scholar] [CrossRef]
- Cadena, C.D.; Kozak, K.H.; Gómez, J.P.; Parra, J.L.; McCain, C.M.; Bowie, R.C.K.; Carnaval, A.C.; Moritz, C.; Rahbek, C.; Roberts, T.E.; et al. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R. Soc. B 2012, 279, 194–201. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef] [Green Version]
- Kindler, C.; Böhme, W.; Corti, C.; Gvoždík, V.; Jablonski, D.; Jandzik, D.; Metallinou, M.; Široký, P.; Fritz, U. Mitochondrial phylogeography, contact zones and taxonomy of grass snakes (Natrix natrix, N. megalocephala). Zool. Scr. 2013, 42, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Marshall, L.; Perdijk, F.; Dendoncker, N.; Kunin, W.; Roberts, S.; Biesmeijer, J.C. Bumblebees moving up: Shifts in elevation ranges in the Pyrenees over 115 years. Proc. R. Soc. B 2020, 287, 20202201. [Google Scholar] [CrossRef]
- Dupoué, A.; Trochet, A.; Richard, M.; Sorlin, M.; Guillon, M.; Teuliere-Quillet, J.; Vallé, C.; Rault, C.; Berroneau, M.; Berroneau, M.; et al. Genetic and demographic trends from rear to leading edge are explained by climate and forest cover in a cold-adapted ectotherm. Divers. Distrib. 2020. [Google Scholar] [CrossRef]
- Gifford, M.E.; Kozak, K.H. Islands in the sky or squeezed at the top? Ecological causes of elevational range limits in montane salamanders. Ecography 2012, 35, 193–203. [Google Scholar] [CrossRef]
- Wiens, J.J.; Camacho, A.; Goldberg, A.; Jezkova, T.; Kaplan, M.E.; Lambert, S.M.; Miller, E.C.; Streicher, J.W.; Walls, R.L. Climate-change, extinction, and Sky Island biogeography in a montane lizard. Mol. Ecol. 2019, 28, 2610–2624. [Google Scholar] [CrossRef]
- Monge, C.; Leon-Velarde, F. Physiological adaptation to high altitude: Oxygen transport in mammals and birds. Physiol. Rev. 1991, 71, 1135–1172. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, J.C.; Beamonte-Barrientos, R.; Bastiaans, E.; Guevara-Fiore, P.; Quintana, E.; Fajardo, V. A mountain or a plateau? Hematological traits vary nonlinearly with altitude in a highland lizard. Physiol. Biochem. Zool. 2017, 90, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, N.; Zhu, Q.; Gaur, U.; Gu, T.; Li, D. High-altitude adaptation of Tibetan chicken from MT-COI and ATP-6 perspective. Mitochondrial DNA A 2016, 27, 3280–3288. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Cruz, M.V.-S.; A Lararesendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal. Niches Sci. 2010, 328, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, J.C.; Van Buskirk, J. Adaptation to elevation but limited local adaptation in an amphibian. Evolution 2020. [Google Scholar] [CrossRef]
- Brown, J.H.; Stevens, G.C.; Kaufman, D.M. The geographic range: Size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996, 27, 597–623. [Google Scholar] [CrossRef] [Green Version]
- Tingley, R.; Vallinoto, M.; Sequeira, F.; Kearney, M.R. Realized niche shift during a global biological invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 10233–10238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef]
- Pottier, G. Plan National d’Actions en Faveur des Lézards des Pyrénées; Nature Midi-Pyrénées: Bagnères de Bigorre, France, 2012; p. 125. [Google Scholar]
- Berroneau, M. Atlas des Amphibiens et Reptiles d’Aquitaine; Cistude Nature: Le Haillan, Gironde, 2014; 257p. [Google Scholar]
- Datcharry, R. Détectabilité en Montagne de Deux Lézards Rupicoles: Le Lézard des Murailles (Podarcis muralis) vs. le Lézard de Bonnal (Iberolacerta bonnali). Mémoire de Stage de Master 2 GBAT; Université Paul Sabatier: Toulouse, France, 2014. [Google Scholar]
- Aguado, S.; Braña, F. Thermoregulation in a cold-adapted species (Cyren’s Rock Lizard, Iberolacerta cyreni): Influence of thermal environment and associated costs. Can. J. Zool. 2014, 92, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Monasterio, C.; Shoo, L.P.; Salvador, A.; Siliceo, I.; Díaz, J.A. Thermal constraints on embryonic development as a proximate cause for elevational range limits in two Mediterranean lacertid lizards. Ecography 2011, 34, 1030–1039. [Google Scholar] [CrossRef]
- Monasterio, C.; Verdú-Ricoy, J.; Salvador, A.; Díaz, J.A. Living at the edge: Lower success of eggs and hatchlings at lower elevation may shape range limits in an alpine lizard. Biol. J. Linn. Soc. 2016, 118, 829–841. [Google Scholar] [CrossRef]
- Osojnik, N.; Žagar, A.; Carretero, M.A.; García-Muñoz, E.; Vrezec, A. Ecophysiological dissimilarities of two sympatric lizards. Herpetologica 2013, 69, 445–454. [Google Scholar] [CrossRef]
- Žagar, A.; Carretero, M.A.; Marguč, D.; Simčič, T.; Vrezec, A. A metabolic syndrome in terrestrial ectotherms with different elevational and distribution patterns. Ecography 2018, 41, 1728–1739. [Google Scholar] [CrossRef] [Green Version]
- Carranza, S.; Arnold, E.N.; Amat, F. DNA phylogeny of Lacerta (Iberolacerta) and other lacertine lizards (Reptilia: Lacertidae): Did competition cause long-term mountain restriction? Syst. Biodivers. 2004, 2, 57–77. [Google Scholar] [CrossRef] [Green Version]
- Žagar, A.; Carretero, M.A.; Osojnik, N.; Sillero, N.; Vrezec, A. A place in the sun: Interspecific interference affects thermoregulation in coexisting lizards. Behav. Ecol. Sociobiol. 2015, 69, 1127–1137. [Google Scholar] [CrossRef]
- Žagar, A.; Carretero, M.A.; Vrezec, A.; Drašler, K.; Kaliontzopoulou, A. Towards a functional understanding of species coexistence: Ecomorphological variation in relation to whole-organism performance in two sympatric lizards. Funct. Ecol. 2017, 31, 1780–1791. [Google Scholar] [CrossRef] [Green Version]
- Monasterio, C.; Salvador, A.; Diaz, J.A. Competition with wall lizards does not explain the alpine confinement of Iberian rock lizards: An experimental approach. Zoology 2010, 113, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Cordero, G.A.; Karnatz, M.L.; Svendsen, J.C.; Gangloff, E.J. Effects of low-oxygen conditions on embryo growth in the painted turtle. Chrysemys. Picta. Integr. Zool. 2017, 12, 148–156. [Google Scholar] [CrossRef]
- Kouyoumdjian, L.; Gangloff, E.J.; Souchet, J.; Cordero, G.A.; Dupoue, A.; Aubret, F. Transplanting gravid lizards to high elevation alters maternal and embryonic oxygen physiology, but not reproductive success or hatchling phenotype. J. Exp. Biol. 2019, 222, jeb206839. [Google Scholar] [CrossRef] [Green Version]
- Mallard, F. Programme les Sentinelles du Climat–Tome IX: Connaitre et Comprendre pour Protéger les Espèces Animales et Végétales face au Changement Climatique; Cistude Nature: Le Haillan, Gironde, 2020; 822p. [Google Scholar]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Bravo, D.N.; Araújo, M.B.; Lasanta, T.; Moreno, J.I.L. Climate change in Mediterranean mountains during the 21st century. AMBIO J. Hum. Environ. 2008, 37, 280–285. [Google Scholar] [CrossRef]
- Walters, R.J.; Blanckenhorn, W.U.; Berger, D. Forecasting extinction risk of ectotherms under climate warming: An evolutionary perspective. Funct. Ecol. 2012, 26, 1324–1338. [Google Scholar] [CrossRef] [Green Version]
- Beninde, J.; Feldmeier, S.; Veith, M.; Hochkirch, A. Admixture of hybrid swarms of native and introduced lizards in cities is determined by the cityscape structure and invasion history. Proc. R. Soc. B 2018, 285, 20180143. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.M.; Gist, D.H.; Taylor, D.H. Home range ecology of an introduced population of the European wall lizard Podarcis muralis (Lacertilia; Lacertidae) in Cincinnati, Ohio. Am. Midl. Nat. 1995, 133, 344–359. [Google Scholar] [CrossRef]
- While, G.M.; Williamson, J.; Prescott, G.; Horvathova, T.; Fresnillo, B.; Beeton, N.J.; Halliwell, B.; Michaelides, S.; Uller, T. Adaptive responses to cool climate promotes persistence of a non-native lizard. Proc. R. Soc. B 2015, 282, 20142638. [Google Scholar] [CrossRef] [PubMed]
- Hedeen, S.E.; Hedeen, D.L. Railway-aided dispersal of an introduced Podarcis muralis population. Herp. Rev. 1999, 30, 57. [Google Scholar]
- Schaefer, J.; Walters, A. Metabolic cold adaptation and developmental plasticity in metabolic rates among species in the Fundulus notatus species complex. Funct. Ecol. 2010, 24, 1087–1094. [Google Scholar] [CrossRef] [Green Version]
- Chown, S.L.; Gaston, K.J. Exploring links between physiology and ecology at macro-scales: The role of respiratory metabolism in insects. Biol. Rev. 1999, 74, 87–120. [Google Scholar] [CrossRef]
- Lardies, M.A.; Bacigalupe, L.D.; Bozinovic, F. Testing the metabolic cold adaptation hypothesis: An intraspecific latitudinal comparison in a common woodlouse. Evol. Ecol. Res. 2004, 6, 567–578. [Google Scholar]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Costantini, D. Oxidative stress ecology and the d-ROMs test: Facts, misfacts and an appraisal of a decade’s work. Behav. Ecol. Sociobiol. 2016, 70, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangloff, E.J.; Telemeco, R.S. High temperature, oxygen, and performance: Insights from reptiles and amphibians. Integr. Comp. Biol. 2018, 58, 9–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangloff, E.J.; Sorlin, M.; Cordero, G.A.; Souchet, J.; Aubret, F. Lizards at the peak: Physiological plasticity does not maintain performance in lizards transplanted to high altitude. Physiol. Biochem. Zool. 2019, 92, 189–200. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Ma, L.; Huebner, C.; Sun, B.; Li, S. Embryonic and post-embryonic responses to high-elevation hypoxia in a low-elevation lizard. Integr. Zool. 2020, 15, 338–348. [Google Scholar] [CrossRef]
- Arribas, O.J. Habitat selection, thermoregulation and activity of the Pyrenean Rock Lizard Iberolacerta bonnali (Lantz, 1927). Herpetozoa 2009, 22, 146–166. [Google Scholar]
- Arribas, O.J. Lagartija pirenaica–Iberolacerta bonnali. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015. [Google Scholar]
- Galán, P.; Arribas, O. Reproductive characteristics of the Pyrenean high-mountain lizards: Iberolacerta aranica (Arribas, 1993), I. aurelioi (Arribas, 1994) and I. bonnali (Lantz, 1927). Anim. Biol. 2005, 55, 163–190. [Google Scholar] [CrossRef] [Green Version]
- Pottier, G.; Arthur, C.-P.; Weber, L.; Cheylan, M. Répartition des lézards du genre Iberolacerta Arribas, 1997 (Sauria: Lacertidae) en France. 3/3: Le Lézard de Bonnal, Iberolacerta bonnali (Lantz, 1927). Bull. Soc. Herp. Fr. 2013, 148, 425–450. [Google Scholar]
- Sinervo, B.; Hedges, R.; Adolph, S.C. Decreased sprint speed as a cost of reproduction in the lizard Sceloporus occidentalis: Variation among populations. J. Exp. Biol. 1991, 155, 323–336. [Google Scholar] [CrossRef]
- Díaz de la Vega-Pérez, A.H.; Barrios-Montiel, R.; Jiménez-Arcos, V.H.; Bautista, A.; Bastiaans, E. High-mountain altitudinal gradient influences thermal ecology of the Mesquite Lizard (Sceloporus grammicus). Can. J. Zool. 2019, 97, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Mathies, T.; Andrews, R. Influence of pregnancy on the thermal biology of the lizard, Sceloporus jarrovi: Why do pregnant females exhibit low body temperatures? Funct. Ecol. 1997, 11, 498–507. [Google Scholar] [CrossRef]
- Blomberg, S.; Shine, R. Reptiles. In Ecological Census Techniques: A Handbook, 2nd ed.; Sutherland, W.J., Ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 297–307. [Google Scholar]
- Fitzgerald, L.A. Finding and Capturing Reptiles. In Reptile Biodiversity: Standard Methods for Inventory and Monitoring; McDiarmid, R.W., Foster, M.S., Guyer, C., Chernoff, N., Gibbons, J.W., Eds.; University of California Press: Oakland, CA, USA, 2012; pp. 77–88. [Google Scholar]
- Vervust, B.; Van Damme, R. Marking lizards by heat branding. Herp. Rev. 2009, 40, 173. [Google Scholar]
- Bouverot, P. Adaptation to Altitude-Hypoxia in Vertebrates; Spring: Berlin, Germany, 1985; p. 176. [Google Scholar]
- Ewalts, M.; Dawkins, T.; Friend, A.T. Hypoxia research: To control or not to control? That is the question. J. Physiol. 2021, 599, 2141–2142. [Google Scholar] [CrossRef] [PubMed]
- Luna, S.; Font, E. Use of an infrared thermographic camera to measure field body temperatures of small lacertid lizards. Herp. Rev. 2013, 44, 59–62. [Google Scholar]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. A 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Diele-Viegas, L.M.; Gangloff, E.J.; Hall, J.M.; Halpern, B.; Massey, M.D.; Rödder, D.; Rollinson, N.; Spears, S.; Sun, B.J.; et al. The thermal ecology and physiology of reptiles and amphibians: A user’s guide. J. Exp. Zool. A 2020. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Mencia, A.; Perez-Mellado, V. The peak of thermoregulation effectiveness: Thermal biology of the Pyrenean rock lizard, Iberolacerta bonnali (Squamata, Lacertidae). J. Therm. Biol. 2016, 56, 77–83. [Google Scholar] [CrossRef]
- Brown, D. Tracker: Video Analysis and Modeling Tool; Version 5.1.3; 2019; Available online: https://physlets.org/tracker/ (accessed on 1 April 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Soft. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.3.3; 2019; Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 April 2019).
- Bates, D.M.; Maechler, M.; Bolker, B.M.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; p. 213. [Google Scholar]
- Stoffel, M.A.; Nakagawa, S.; Schielzeth, H.; Goslee, S. RptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Method Ecol. Evol. 2017, 8, 1639–1644. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.C. Temperature and hypoxia in ectothermic tetrapods. J. Therm. Biol. 2007, 32, 125–133. [Google Scholar] [CrossRef]
- Irschick, D.J.; Garland, T., Jr. Integrating function and ecology in studies of adaptation: Investigations of locomotor capacity as a model system. Ann. Rev. Ecol. Syst. 2001, 32, 367–396. [Google Scholar] [CrossRef] [Green Version]
- Miles, D.B. The race goes to the swift: Fitness consequences of variation in sprint performance in juvenile lizards. Evol. Ecol. Res. 2004, 6, 63–75. [Google Scholar]
- Barja, G. Aging in vertebrates, and the effect of caloric restriction: A mitochondrial free radical production-DNA damage mechanism? Biol. Rev. Camb. Philos. Soc. 2004, 79, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Donati, A.; Girardis, M. Oxygen in the critically ill: Friend or foe? Curr. Opin. Anesthesio. 2018, 31, 129–135. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Seibel, B.A.; Deutsch, C. Oxygen supply capacity in animals evolves to meet maximum demand at the current oxygen partial pressure regardless of size or temperature. J. Exp. Biol. 2020, 223, jeb210492. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.C.; VandenBrooks, J.M.; Riley, A.; Telemeco, R.S.; Angilletta, M.J., Jr. Oxygen supply did not affect how lizards responded to thermal stress. Integr. Zool. 2018, 13, 428–436. [Google Scholar] [CrossRef]
- Hicks, J.W.; Wood, S.C. Temperature regulation in lizards: Effects of hypoxia. Am. J. Physiol. Reg. Integr. Comp. Physiol. 1985, 248, R595–R600. [Google Scholar] [CrossRef]
- He, J.; Xiu, M.; Tang, X.; Wang, N.; Xin, Y.; Li, W.; Chen, Q. Thermoregulatory and metabolic responses to hypoxia in the oviparous lizard, Phrynocephalus przewalskii. Comp. Biochem. Physiol. A 2013, 165, 207–213. [Google Scholar] [CrossRef]
- Shea, T.K.; DuBois, P.M.; Claunch, N.M.; Murphey, N.E.; Rucker, K.A.; Brewster, R.A.; Taylor, E.N. Oxygen concentration affects upper thermal tolerance in a terrestrial vertebrate. Comp. Biochem. Physiol. A 2016, 199, 87–94. [Google Scholar] [CrossRef]
- McClelland, G.B.; Scott, G.R. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol. 2019, 81, 561–583. [Google Scholar] [CrossRef]
- Voituron, Y.; Boel, M.; Roussel, D. Mitochondrial threshold for H2O2 release in skeletal muscle of mammals. Mitochondrion 2020, 54, 85–91. [Google Scholar] [CrossRef]
- Koch, R.E.; Buchanan, K.L.; Casagrande, S.; Crino, O.; Dowling, D.K.; Hill, G.E.; Hood, W.R.; McKenzie, M.; Mariette, M.M.; Noble, D.W.A.; et al. Integrating mitochondrial aerobic metabolism into ecology and evolution. Trends Ecol. Evol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, A.L.; Brown, J.H. Biogeography; Sinauer Associates: Sunderland, MA, USA, 2010; p. 560. [Google Scholar]
- Jankowski, J.E.; Robinson, S.K.; Levey, D.J. Squeezed at the top: Interspecific aggression may constrain elevational ranges in tropical birds. Ecology 2010, 91, 1877–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, Z.; Mencia, A.; Perez-Mellado, V. Are mountain habitats becoming more suitable for generalist than cold-adapted lizards thermoregulation? PeerJ 2016, 4, e2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monasterio, C.; Salvador, A.; Iraeta, P.; Díaz, J.A. The effects of thermal biology and refuge availability on the restricted distribution of an alpine lizard. J. Biogeogr. 2009, 36, 1673–1684. [Google Scholar] [CrossRef]
- Ortega, Z.; Mencía, A.; Pérez-Mellado, V. Behavioral buffering of global warming in a cold-adapted lizard. Ecol. Evol. 2016, 6, 4582–4590. [Google Scholar] [CrossRef] [Green Version]
- Levis, N.A.; Pfennig, D.W. Evaluating ‘Plasticity-first’ evolution in nature: Key criteria and empirical approaches. Trends Ecol. Evol. 2016, 31, 563–574. [Google Scholar] [CrossRef]
- Lande, R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 2009, 22, 1435–1446. [Google Scholar] [CrossRef]
- Žagar, A.; Simčič, T.; Carretero, M.A.; Vrezec, A. The role of metabolism in understanding the altitudinal segregation pattern of two potentially interacting lizards. Comp. Biochem. Physiol. A 2015, 179, 1–6. [Google Scholar] [CrossRef]
- Megía-Palma, R.; Jiménez-Robles, O.; Hernández-Agüero, J.A.; De la Riva, I. Plasticity of haemoglobin concentration and thermoregulation in a mountain lizard. J. Therm. Biol. 2020, 92, 102656. [Google Scholar] [CrossRef]
- Jiang, Z.-W.; Ma, L.; Mi, C.-R.; Du, W.-G. Effects of hypoxia on the thermal physiology of a high-elevation lizard: Implications for upslope-shifting species. Biol. Lett. 2021, 17, 20200873. [Google Scholar] [CrossRef]
- Cordero, G.A.; Andersson, B.A.; Souchet, J.; Micheli, G.; Noble, D.W.A.; Gangloff, E.J.; Uller, T.; Aubret, F. Physiological plasticity in lizard embryos exposed to high-altitude hypoxia. J. Exp. Zool. A 2017, 327, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Souchet, J.; Bossu, C.; Darnet, E.; Le Chevalier, H.; Poignet, M.; Trochet, A.; Bertrand, R.; Calvez, O.; Martinez-Silvestre, A.; Mossoll-Torres, M.; et al. High temperatures limit developmental resilience to high-elevation hypoxia in the snake Natrix maura (Squamata: Colubridae). Biol. J. Linn. Soc. 2020, 132, 116–133. [Google Scholar] [CrossRef]
- Souchet, J.; Gangloff, E.J.; Micheli, G.; Bossu, C.; Trochet, A.; Bertrand, R.; Clobert, J.; Calvez, O.; Martinez-Silvestre, A.; Darnet, E.; et al. High-elevation hypoxia impacts perinatal physiology and performance in a potential montane colonizer. Integr. Zool. 2020, 15, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F. High-altitude adaptation: Mechanistic insights from integrated genomics and physiology. Mol. Biol. Evol. 2021. [Google Scholar] [CrossRef]
- Li, J.T.; Gao, Y.D.; Xie, L.; Deng, C.; Shi, P.; Guan, M.L.; Huang, S.; Ren, J.L.; Wu, D.D.; Ding, L.; et al. Comparative genomic investigation of high-elevation adaptation in ectothermic snakes. Proc. Natl. Acad. Sci. USA 2018, 115, 8406–8411. [Google Scholar] [CrossRef] [Green Version]



| Source of Variation | Test Statistics |
|---|---|
| Temperature | |
| F (dfn, dfd) | 114.8 (4, 152) |
| Pr > F | <0.001 |
| Treatment | |
| F (dfn, dfd) | 5.13 (1, 37) |
| Pr > F | 0.030 |
| Temperature × Treatment | |
| F (dfn, dfd) | 2.84 (4, 152) |
| Pr > F | 0.026 |
| Snout-vent length | |
| F (dfn, dfd) | 4.02 (1, 37) |
| Pr > F | 0.052 |
| Temperature (°C) | Low Elevation (SE) | High Elevation (SE) | Difference (SE) | Significance Test |
|---|---|---|---|---|
| 15 | −0.176 (0.02) | −0.188 (0.02) | 0.012 (0.29) | t171 = 0.43 p = 0.668 |
| 22 | 0.022 (0.02) | 0.013 (0.02) | 0.009 (0.29) | t171 = 0.33 p = 0.744 |
| 29 | 0.054 (0.02) | 0.113 (0.02) | −0.059 (0.29) | t171 = −2.068 p = 0.040 |
| 32 | 0.103 (0.02) | 0.171 (0.02) | −0.068 (0.29) | t171 = 0.−2.39 p = 0.018 |
| 35 | 0.134 (0.02) | 0.212 (0.02) | −0.079 (0.29) | t171 = −2.77 p = 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangloff, E.J.; Spears, S.; Kouyoumdjian, L.; Pettit, C.; Aubret, F. Does Hyperoxia Restrict Pyrenean Rock Lizards Iberolacerta bonnali to High Elevations? Diversity 2021, 13, 200. https://doi.org/10.3390/d13050200
Gangloff EJ, Spears S, Kouyoumdjian L, Pettit C, Aubret F. Does Hyperoxia Restrict Pyrenean Rock Lizards Iberolacerta bonnali to High Elevations? Diversity. 2021; 13(5):200. https://doi.org/10.3390/d13050200
Chicago/Turabian StyleGangloff, Eric J., Sierra Spears, Laura Kouyoumdjian, Ciara Pettit, and Fabien Aubret. 2021. "Does Hyperoxia Restrict Pyrenean Rock Lizards Iberolacerta bonnali to High Elevations?" Diversity 13, no. 5: 200. https://doi.org/10.3390/d13050200
APA StyleGangloff, E. J., Spears, S., Kouyoumdjian, L., Pettit, C., & Aubret, F. (2021). Does Hyperoxia Restrict Pyrenean Rock Lizards Iberolacerta bonnali to High Elevations? Diversity, 13(5), 200. https://doi.org/10.3390/d13050200