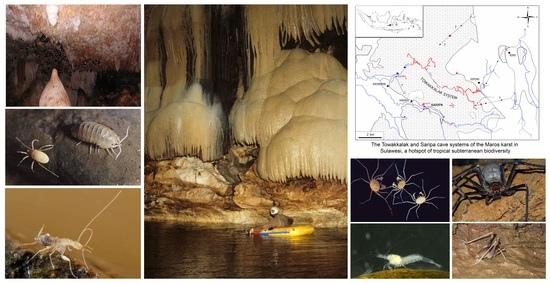
The Towakkalak System, A Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia
Abstract
:1. Introduction and Context
1.1. Geographical Setting
1.2. Archeology
1.3. Geology
1.4. Geomorphology
1.5. Caves
2. Material and Methods
2.1. The Towakkalak System (Figures 2B–E and 3)
2.2. The Saripa System
2.3. Sampling
3. Results
3.1. Aquatic Fauna
- -
- Caridea (Figure 5B): The shrimps found in the systems of interest belong to four genera: Caridina H. Milne-Edwards, 1837 (with 1 stygobiotic species restricted to Towakkalak system and 1 stygophilic species); Marosina, Cai and Ng, 2005 (endemic genus with two stygobiotic species only known from the Maros karst, one of which is limited to Gua Salukkan Kallang), Parisia Holthuis, 1956 (endemic genus with one rare troglobiotic species only found in Gua Tanette), and Macrobrachium lar, a large size stygophile widespread in Pacific and Indian Ocean islands [62,63,64]. These shrimps often live as large populations in springs, streams, and puddles. Their diversity in the Maros karst is reminiscent of the radiation of the genus Caridina in the lakes of Sulawesi [65], which is not matched elsewhere in tropical caves in Asia and obviously calls for further sampling. The proneness to colonize cave habitats repeats in several clades of the worldwide distributed family Atyidae [66] but is particularly marked in the Maros karst, which has the highest number of troglobiotic species in Australasia and the highest level of troglomorphy with its two Marosina species.
- -
- Brachyura (Figure 5C,D): Crabs are frequent in the Maros caves, with four species. A total of three of them belong to the speciose sundaic genus Parathelphusa H. Milne-Edwards, 1853, of which P. sorella, a cave-obligate species with reduced eyes and that is restricted to Saripa cave, where it is rather common [67]. The most remarkable crab species of Maros is the small Cancrocaeca xenomorpha, which is blind and with very long and thin legs [68]. The monospecific genus Cancrocaeca Ng, 1991, belongs to a family of mostly marine species, but the Maros species only lives in freshwater, both in the Towakkalak and Saripa systems, where it occurs sporadically in standing water puddles. A second species of Hymenosomatidae discovered more recently in a cave of the Sangkulirang karst of Kalimantan, Guaplax denticulata Naruse, Ng and Guinot, 2008, also lives in freshwater. Both differ from the third cave Hymenosomatidae of the region, Sulaplax ensifer Naruse, Ng and Guinot, 2008, from Muna Island in Southeast Sulawesi, which lives in brackish water.
3.2. Terrestrial Fauna
- -
- Speocera caeca: This troglobiont is widely represented in the caves of the two systems. It is the only blind species of the speciose tropical genus Speocera Berland, 1914, which includes several other cave species [73].
- -
- The species cf. Amauropelma sp.: It is a troglobiotic spider that has been found in several caves of the two systems. By its reduced and unpigmented eyes, pale body color, and rather large size, it is reminiscent of Amauropelma matakecil Miller and Rahmadi, 2012, that was recently described from caves of Central Java [74].
- -
- Psiloderces leclerci: The speciose Southeast Asia genus Psiloderces Simon, 1892, is known by one species in our study, P. leclerci, from one cave and one surface site. The latter form is ‘much deeper in color’ and has shorter legs than that of the cave [75], suggesting a possible separate species status. Psiloderces leclerci belongs to the speciose genus Psiloderces of the South Asian family Psilodercidae, widespread on Sunda island with a few other cave species.
- -
- Spermophora maros: The genus Spermophora Hentz, 1841, comprises 45 species that are widely distributed in tropical regions around the world [53] but that are rare in caves. Spermophora maros is the only species of the Sunda Islands to have reduced unpigmented eyes, long legs, and whitish coloration [76].
- -
- Uthina mimpi: The 17 species of the genus Uthina Simon, 1893 [53], mostly live in forest litter. Uthina mimpi is, however, a weakly modified species of pale coloration, slightly reduced eyes, and slender legs that seems to be fully troglobiont. In addition, two other Uthina species of less clear ecological status, the widespread troglophilic Uthina luzonica Simon, 1893, and the Maros endemic U. sulawesiensis Yao and Li, 2016, probably exist in our study area [77].
4. Discussion and Conclusions
4.1. Limitations of The Checklist
4.1.1. Taxonomy
4.1.2. Habitats
4.1.3. Sampling Methods
4.1.4. Species Ecological Status
4.2. Cave Fauna Features of The Towakkalak and Saripa Systems
4.2.1. Species Richness
4.2.2. Endemism
4.2.3. Shared Diversity Features
4.2.4. Troglomorphy
4.2.5. Guano
4.2.6. Invasives
4.3. Conservation Issues
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deharveng, L.; Bedos, A. Diversity of Terrestrial Invertebrates in Subterranean Habitats. In Cave Ecology, Ecological Studies 235; Moldovan, O.T., Kovác, L., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 107–172. [Google Scholar]
- Deharveng, L.; Bedos, A. Chapter 18—Diversity Patterns in the Tropics. In Encyclopedia of Caves; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 146–162. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Caves Karst Studies 2000, 62, 11–17. [Google Scholar]
- Deharveng, L.; Bedos, A. The cave fauna of Southeast Asia. Origin, evolution and ecology. In Ecosystems of the World 30. Subterranean Ecosystems; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 603–632. [Google Scholar]
- Suhardjono, Y.R.; Ubaidillah, R. (Eds.) Fauna Karst dan gua Maros, Sulawesi Selatan; LIPI Press: Jakarta, Indonesia, 2012; 258p. [Google Scholar]
- Balazs, D. Karst Regions in Indonesia. Karszt Es Barlang 1968, 5, 3–61. [Google Scholar]
- Arsyad, M.; Sulistiawaty, U.; Tiwow, V.A. Analysis of Characteristics and Classification of Rainfall in the Maros Karst Region, South Sulawesi. In Proceedings of the International Seminar on Mathematic, Science and Computer Education, Bandung, Indonesia, 15 October 2016. [Google Scholar]
- Sarasin, P.; Sarasin, F. Die Toala-Hoehlen von Lamontjong. Versuch Einer Anthropologie der Insel Celebes; C.W. Kriedel’s Verlag: Wiesbaden, Germany, 1905. [Google Scholar]
- Van Heekeren, H.R. Rock-paintings and other prehistoric discoveries near Maros (South West Celebes). Laporan Tahunan 1952, 22–35. [Google Scholar]
- Glover, I.C.; Sinha, P. Changes in stone tool use 10,000 years ago: A microwear analysis of flakes with use gloss from Leang Burung 2 and Ulu Leang 1 caves, Sulawesi, Indonesia. Mod. Quat. Res. S. E. Asia 1984, 8, 137–164. [Google Scholar]
- Aubert, M.; Brumm, A.; Ramli, M.; Sutikna, T.; Saptomo, E.W.; Hakim, B.; Morwood, M.J.; van den Bergh, G.D.; Kinsley, L.; Dosseto, A. Pleistocene cave art from Sulawesi, Indonesia. Nature 2014, 514, 223–227. [Google Scholar] [CrossRef]
- Brumm, A.; Oktaviana, A.A.; Burhan, B.; Hakim, B.; Lebe, R.; Zhao, J.X.; Sulistyarto, P.H.; Ririmasse, M.; Adhityatama, S.; Sumantri, I.; et al. Oldest cave art found in Sulawesi. Sci. Adv. 2021, 7, eabd4648. [Google Scholar] [CrossRef]
- Huntley, J.; Aubert, M.; Oktaviana, A.A.; Lebe, R.; Hakim, B.; Burhan, B.; Muhammad Aksa, L.; Made Geria, I.; Ramli, M.; Siagian, L.; et al. The effects of climate change on the Pleistocene rock art of Sulawesi. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brouquisse, F. 13. Cadre géologique. In Expédition Thaï-Maros 85, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1986; pp. 101–118. [Google Scholar]
- Wallace, A.R. The Malay Archipelago: The Land of the Orang-Utan and the Bird of Paradise: A Narrative of Travel with Studies of Man and Nature; Macmillan and Co.: London, UK, 1890. [Google Scholar]
- Sunartadirdja, M.A.; Lehmann, H. Der tropische Karst von Maros und Nord-Bone in SW-Celebes (Sulawesi). Z. Geomorph. 1960, 4 (Suppl. 2), 49–65. [Google Scholar]
- Crabtree, S.; Friederich, H. The Caves of the Bau District, Sarawak. Cave Sci. 1982, 9, 83–93. [Google Scholar]
- Kusch, H. Speläologische Forschungen auf der Insel Sulawesi (Celebes, Indonesien) zwischen 1857 und 1977. Die Höhle 1981, 32, 91–102. [Google Scholar]
- Deharveng, L.; Bedos, A. 10. Les cavités des environs de Bantimurung. In Expédition Thaï-Maros 85, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1986; pp. 81–95. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expédition Thaï-Maros 85, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1986; pp. 1–215. [Google Scholar]
- De Vivo, A.; Campion, N.; Menin, A.; Viviani, F. Vecchie storie Indonesiane. Speleologia 1992, 27, 32–41. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expédition Thaï-Maros 86, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1987; pp. 1–174. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expéditions de l’A.P.S. en Asie du Sud-Est. Travaux Scientifiques—1; APS: Toulouse, France, 1988; pp. 1–52. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expéditions Maros 88-Maros 89, Rapport Spéléologique; APS: Toulouse, France, 1990; pp. 1–51. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expédition Indonésie 90, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1992; pp. 1–104. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expédition Maros 94, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1997; pp. 1–40. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Indonésie 92, Rapport Spéléologique; APS: Toulouse, France, 2001; pp. 1–67. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Expédition Maros 99, Rapport Spéléologique; APS: Toulouse, France, 2002; pp. 1–39. [Google Scholar]
- Association Pyrénéenne de Spéléologie. Maros 2001—Indonésie—Sulawesi Selatan, Rapport Spéléologique, unpublished, no date. 25p.
- Brouquisse, F.; Deharveng, L.; Laumanns, M. Indonesia 1985–2001 Expeditions of the Association Pyrénéenne de Spéléologie. Berl. Höhlenkundliche Ber. 2015, 59, 1–197. [Google Scholar]
- Acintyacunyata Speleological Club. Laporan Ekspedisi Maros 1989—Sulawesi Selatan, Unpublished, no date. 1–146.
- Suhardjono, Y.R.; Rahmadi, C.; Nugroho, H.; Wiantoro, S. Bab 2 Karst dan Gua. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 13–52. [Google Scholar]
- Bedos, A.; Deharveng, L.; Deharveng, L.; Leclerc, P.; Rigal, D.; Solier, P. 3. Résultats spéléologiques. In Expéditions Maros 88-Maros 89, Rapport Spéléologique; APS: Toulouse, France, 1990; pp. 15–48. [Google Scholar]
- Brouquisse, F.; Lacas, M.; Rigal, D. 5. Sulawesi: Résultats spéléologiques. In Expédition Indonésie 90, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1992; pp. 37–82. [Google Scholar]
- Bedos, A.; Brouquisse, F.; Deharveng, L.; Leclerc, P.; Rigal, D. 4. Grandes Cavités du Karst de Maros. In Indonésie 92, Rapport Spéléologique; APS: Toulouse, France, 2001; pp. 39–45. [Google Scholar]
- Brehier, F. Reconnaissance de quelques siphons du karst de Maros. Maros 2001—Indonésie—Sulawesi Selatan, Rapport Spéléologique, Unpublished. 16–20.
- Rigal, D. Leang Assuloang. Maros 2001—Indonésie—Sulawesi Selatan, Rapport Spéléologique, Unpublished. 7–10.
- Rigal, D.; Lacas, M. 3. Nouvelles découvertes sur le karst de Maros. In Indonésie 92, Rapport Spéléologique; APS: Toulouse, France, 2001; pp. 15–37. [Google Scholar]
- Brouquisse, F.; Brouquisse, R. 4. Résultats spéléologiques. In Expédition Maros 94, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1997; pp. 14–28. [Google Scholar]
- Brouquisse, F. 9. Le secteur de Kappang et le réseau de Gua Salukkan Kallang. In Expédition Thaï-Maros 85, Rapport Spéléologique et scientifique; APS: Toulouse, France, 1986; pp. 68–80. [Google Scholar]
- Rigal, D. Gua Salukkan Kallang, karst de Maros, Célèbes Sud, Indonésie. Spelunca 1987, 28, 32–39. [Google Scholar]
- Brouquisse, F.; Rigal, D. 6. Résultats spéléologiques Sulawesi. In Expédition Thaï–Maros 86, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1987; pp. 47–74. [Google Scholar]
- Brouquisse, F.; Dalger, D. 8. Hydrogéochimie. In Expédition Thaï-Maros 86, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1987; pp. 85–96. [Google Scholar]
- Deharveng, L. 10. Programme zoologique: Bilan général et principaux résultats. In Expédition Thaï-Maros 86, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1987; pp. 111–116. [Google Scholar]
- Galletti, I. Nota biospeleologica della spedizione Sulawesi ‘94. Speleologia Iblea 1996, 4, 98–101. [Google Scholar]
- Deharveng, L.; Bedos, A. Salukkan Kallang, Indonesia: Biospeleology. In Encyclopedia of Cave and Karst Science; Gunn, J., Ed.; Fitzroy Dearborn: London, UK, 2004; pp. 631–633. [Google Scholar]
- Suhardjono, Y.R. Review of Biospeleology in Sulawesi Island. In Proceedings of the International Symposium on The Ecology and Limnology of the Malili Lakes, Bogor, Indonesia, 20–23 March 2006; pp. 29–38. [Google Scholar]
- Chapman, P. The ecology of caves in the Gunung Mulu National Park, Sarawak. Trans. British Cave Research Assoc. 1982, 9, 142–162. [Google Scholar]
- Brancelj, A.; Boonyanusith, C.; Watiroyram, S.; Sanoamuang, L.O. The groundwater-dwelling fauna of Southeast Asia. J. Limnol. 2013, 72, e16. [Google Scholar] [CrossRef] [Green Version]
- Kawakatsu, M.; Mitchell, R.W. Two new freshwater cavernicole planarians (Turbellaria, Tricladida, Paludicola) from Sulawesi (Celebes), Indonesia. Spec. Bull. Jpn. Soc. Coleopterol. 1995, 4, 81–104. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. Available online: https://www.marinespecies.orgatVLIZ (accessed on 15 June 2021).
- Harvey, M.S. Whip Spiders of the World, Version 1.0. Western Australian Museum, Perth. 2013. Available online: http://www.museum.wa.gov.au/catalogues/whip-spiders (accessed on 13 June 2021).
- World Spider Catalog. Version 22.5. Natural History Museum Bern. Available online: http://wsc.nmbe.ch (accessed on 5 July 2021).
- Bu, Y.; Souza, M.F.V.R.; Mayoral, J. New and interesting palpigrades (Arachnida, Palpigradi) of the genera Koeneniodes Silvestri, 1913 and Prokoenenia Börner, 1901 from Asia. Zootaxa 2021, 4990, 45–64. [Google Scholar] [CrossRef]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World 1996–2021. Available online: http://www.collembola.org (accessed on 10 June 2021).
- Sendra, A.; Jiménez-Valverde, A.; Rochat, J.; Legros, V.; Gasnier, S.; Cazanove, G. A new and remarkable troglobitic Lepidocampa Oudemans, 1890 species from La Réunion Island, with a discussion on troglobiomorphic adaptations in campodeids (Diplura). Zoologischer Anzeiger 2017, 266, 95–104. [Google Scholar] [CrossRef]
- Faille, A. Les Coléoptères troglobies de l'île de Sulawesi (Indonésie); description du mâle du Paussidae cavernicole Eustra saripaensis Deuve, 2002 (Coleoptera). Bull. Soc. Entomol. Fr. 2010, 115, 375–380. [Google Scholar]
- Deuve, T. Sur une population différenciée de Mateuellus troglobioticus (Deuve, 1990) dans le sud de Sulawesi (Col., Caraboidea, Harpalidae, Pterostichinae, Abacetini). Bull. Soc. Entom. Fr. 2010, 115, 310. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://Orthoptera.SpeciesFile.org (accessed on 5 July 2021).
- Integrated Taxonomic Information System (ITIS). Available online: www.itis.gov (accessed on 4 July 2021).
- Botosaneanu, L. New stygobiontic isopods (Isopoda: Cirolanidae, Anthuridae) from caves in Sulawesi, Indonesia. Bull. Inst. R. Sci. Nat. Belg. 2003, 73, 91–105. [Google Scholar]
- Wowor, D.; Rahmadi, C. Bab 8 Krustasea. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 165–190. [Google Scholar]
- Cai, Y.; Ng, P.K.L. Marosina, a New Genus of Troglobitic Shrimps (Decapoda, Atyidae) from Sulawesi, Indonesia, with Descriptions of Two New Species. Crustaceana 2005, 78, 129–139. [Google Scholar]
- Cai, Y.; Ng, P.K.L. The freshwater shrimps of the genera Caridina and Parisia from karst caves of Sulawesi Selatan, Indonesia, with descriptions of three new species (Crustacea: Decapoda: Caridea: Atyidae. J. Nat. Hist. 2009, 43, 1093–1114. [Google Scholar] [CrossRef]
- von Rintelen, K.; Glaubrecht, M.; Schubart, C.; Wessel, A.; von Rintelen, T. Adaptive radiation and ecological diversification of Sulawesi’s ancient lake shrimps. Evolution 2010, 64, 3287–3299. [Google Scholar] [CrossRef]
- von Rintelen, K.; Page, T.J.; Cai, Y.; Roe, K.; Stelbrink, B.; Kuhajda, B.R.; Iliffe, T.M.; Hughes, J.; von Rintelen, T. Drawn to the dark side: A molecular phylogeny of freshwater shrimps (Crustacea: Decapoda: Caridea: Atyidae) reveals frequent cave invasions and challenges current taxonomic hypotheses. Mol. Phylogenet. Evol. 2012, 63, 82–96. [Google Scholar] [CrossRef]
- Chia, O.K.S.; Ng, P.K.L. The freshwater crabs of Sulawesi, with descriptions of two new genera and four new species (Crustacea: Decapoda: Brachyura: Parathelphusidae). Raffles Bull. Zool. 2006, 54, 381–428. [Google Scholar]
- Ng, P.K.L. Cancrocaeca xenomorpha, new genus and species, a blind troglomorphic freshwater hymenosomatid (Crustacea: Decapoda: Brachyura) from Sulawesi, Indonesia. Raffles Bull. Zool. 1991, 39, 59–73. [Google Scholar]
- Hoese, D.F.; Kottelat, M. Bostrychus microphthalmus, a new microphthalmic cavefish from Sulawesi (Teleostei: Gobiidae). Ichthyol. Explor. Freshwaters 2005, 16, 183–191. [Google Scholar]
- Hadiaty, R.K. Bab 5 Ikan. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 89–113. [Google Scholar]
- Proudlove, G. Subterranean Fishes of the World. Available online: https://cavefishes.org.uk (accessed on 14 June 2021).
- Marwoto, R.M.; Isnaningsih, N.R. Bab 6 Molluska. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 115–148. [Google Scholar]
- Brescovit, A.D.; Zampaulo, R.D.A.; Cizauskas, I. The first two blind troglobitic spiders of the genus Ochyrocera from caves in Floresta Nacional de Carajás, state of Pará, Brazil (Araneae, Ochyroceratidae). Zookeys 2021, 1031, 143. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Rahmadi, C. A troglomorphic spider from Java (Araneae, Ctenidae, Amauropelma). Zookeys 2012, 163, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeleman-Reinhold, C.L. The Ochyroceratidae of the Indo-Pacific region (Araneae). Raffles Bull. Zool. 1995, 43 (Suppl. 2), 1–103. [Google Scholar]
- Huber, B.A. Revision of the genus Spermophora Hentz in Southeast Asia and on the Pacific Islands, with descriptions of three new genera (Araneae: Pholcidae). Zool. Meded. 2005, 79, 61–114. [Google Scholar]
- Huber, B.A.; Caspar, K.R.; Eberle, J. New species reveal unexpected interspecific microhabitat diversity in the genus Uthina Simon, 1893 (Araneae: Pholcidae). Invertebr. Syst. 2019, 33, 181–207. [Google Scholar] [CrossRef]
- Abrams, K.M.; Huey, J.A.; Hillyer, M.J.; Humphreys, W.F.; Didham, R.K.; Harvey, M.S. Too hot to handle: Cenozoic aridification drives multiple independent incursions of Schizomida (Hubbardiidae) into hypogean environments. Mol. Phylogenet. Evol. 2019, 139, 106532. [Google Scholar] [CrossRef]
- Rahmadi, C. Bab 9 Arthropoda Gua. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 191–214. [Google Scholar]
- Kraepelin, K. Das Tierreich 8—Scorpiones und Pedipalpi; R. Friedländer und Sohn: Berlin, Germany, 1899; pp. 1–265. [Google Scholar]
- Harvey, M.S.; West, P.L.J. New species of Charon (Amblypygi, Charontidae) from northern Australia and Christmas Island. J. Arachnol. 1998, 26, 273–284. [Google Scholar]
- Rahmadi, C.; Harvey, M.S.; Kojima, J.I. The status of the whip spider subgenus Neocharon (Amblypygi: Charontidae) and the distribution of the genera Charon and Stygophrynus. J. Arachnol. 2011, 39, 223–229. [Google Scholar] [CrossRef]
- Golovatch, S.I.; Geoffroy, J.J.; Mauriès, J.P.; van den Spiegel, D. Review of the millipede genus Eutrichodesmus Silvestri, 1910 (Diplopoda, Polydesmida, Haplodesmidae), with descriptions of new species. Zookeys 2009, 12, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Dalens, H. 12. Données préliminaires sur les Isopodes terrestres récoltés dans les grottes de Sulawesi et des Moluques. In Expédition Thaï-Maros 86, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1987; pp. 129–132. [Google Scholar]
- Suhardjono, Y.R. Bab 11 Ekorpegas. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 227–246. [Google Scholar]
- Deharveng, L.; Suhardjono, Y.R. Pseudosinella maros sp. n., a troglobitic Entomobryidae (Collembola) from Sulawesi Selatan, Indonesia. Rev. Suisse Zool. 2004, 111, 979–984. [Google Scholar] [CrossRef]
- Cipola, N.G.; Oliveira, J.V.L.C.; Bellini, B.C.; Ferreira, A.S.; Lima, E.C.A.; Brito, R.A.; Stievano, L.C.; Souza, P.G.C.; Zeppelini, D. Review of Eyeless Pseudosinella Schäffer (Collembola, Entomobryidae, Lepidocyrtinae) from Brazilian Caves. Insects 2020, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Deharveng, L. 4. La faune souterraine de Batu Lubang. In Expédition Batukarst 88, Rapport Spéléologique et Scientifique; APS: Toulouse, France, 1989; pp. 37–46. [Google Scholar]
- Deharveng, L.; Whitten, T.; Leclerc, P. 5.13. Caves of Papua. In The Ecology of Papua; Marshall, A., Beehler, B., Eds.; Periplus: Budapest, Hungary, 2007; pp. 1064–1082. [Google Scholar]
- Condé, B. Campodéides des grottes des Célèbes (Insectes, Diploures). Mém. Biospéol. 1992, 19, 155–158. [Google Scholar]
- Allegrucci, G.; Trewick, S.A.; Fortunato, A.; Carchini, G.; Sbordoni, V. Cave crickets and cave weta (Orthoptera, Rhaphidophoridae) from the southern end of the World: A molecular phylogeny test of biogeographical hypotheses. J. Orthoptera Res. 2010, 19, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Rheindt, F.E.; Norman, J.A.; Christidis, L. Extensive diversification across islands in the echolocating Aerodramus swiftlets. Raffles Bull. Zool. 2014, 62, 89–99. [Google Scholar]
- Suyanto, A.; Wiantoro, S. Bab 3 Kelelawar. In Fauna Karst dan gua Maros, Sulawesi Selatan; Suhardjono, Y.R., Ubaidillah, R., Eds.; LIPI Press: Jakarta, Indonesia, 2012; pp. 53–76. [Google Scholar]
- Chapman, P. Species diversity in a tropical cave ecosystem. Proc. Univ. Bristol Spelaeol. Soc. 1983, 16, 201–213. [Google Scholar]
- Coiffait, H. Les Coléoptères du sol. Vie et Milieu 1958, 9 (Suppl. 7), 1–204. [Google Scholar]
- Beron, P. Comparative study of the invertebrate cave faunas of Southeast Asia and New Guinea. Hist. Nat. Bulg. 2015, 21, 169–210. [Google Scholar]
- Souza Silva, M.; Ferreira, R.L. The first two hotspots of subterranean biodiversity in South America. Subterr. Biol. 2016, 19, 1–21. [Google Scholar] [CrossRef]
- Trajano, E.; Gallão, J.E.; Bichuette, M.E. Spots of high diversity of troglobites in Brazil: The challenge of measuring subterranean diversity. Biodivers. Conserv. 2016, 25, 1805–1828. [Google Scholar] [CrossRef]
- Jeannel, R.; Racovitza, E.G. Biospeologica XXXIX. Enumération des grottes visitées 1913–1917 (sixième série). Arch. Zool. Exp. Gen. 1918, 57, 203–470. [Google Scholar]
- Deharveng, L.; Lips, J.; Rahmadi, C. Focus on guano. In The Natural History of Santo: Caves and Soils; Bouchet, P., Le Guyader, H., Pascal, O., Eds.; MNHN; IRD; PNI: Paris, France, 2011; pp. 300–305. [Google Scholar]
- Hanitsch, R. On some cave-dwelling Blattids from Celebes. Tijdschr. Entomol. 1932, 75, 264–265. [Google Scholar]







| Taxonomic Group | Taxon | Ecology | Dist | Towakkalak | Saripa | Gen Val |
|---|---|---|---|---|---|---|
| Tricladida: Dugesiidae | Dugesia leclerci Kawakatsu and Mitchell, 1995 | SB | end | 9 | L?,S? | 121 [51] |
| Tricladida: Dugesiidae | Dugesia uenorum Kawakatsu and Mitchell, 1995 | SB? | end * | Pa | 121 [51] | |
| Amphipoda: Bogidiellidae | Bogidiellidae sp. | SB | end | 9 | n.a. | |
| Isopoda: Cirolanidae | Cirolana marosina Botosaneanu, 2003 | SB | end | 12a | S | 146 [51] |
| Caridea: Atyidae | Caridina leclerci Cai and Ng, 2009 | SB | end * | 2,3,9 | 344 [51] | |
| Caridea: Atyidae | Marosina brevirostris Cai and Ng, 2005 | SB | end * | 2 | 2 [51] | |
| Caridea: Atyidae | Marosina longirostris Cai and Ng, 2005 | SB | end | 2,8,9 | L | 2 [51] |
| Caridea: Atyidae | Parisia deharvengi Cai and Ng, 2009 | SB | end * | 9 | 9 [51] | |
| Brachyura: Hymenosomatidae | Cancrocaeca xenomorpha Ng, 1991 | SB | end | 8,9 | L,S | 1 [51] |
| Brachyura: Gecarcinucidae | Parathelphusa sorella Chia and Ng, 2006 | SB | end * | L | 49 [51] | |
| Pisces: Eleotridae | Bostrychus microphthalmus Hoese and Kottelat, 2005 | SB | end | 9 | L | 9 [51] |
| Acari: Leeuwenhoekiidae | Leeuwenhoekiidae sp. (cf.) | TB? | n.a. | L,S | n.a. | |
| Amblypygi: Charinidae | Sarax sp. | TB | end? | 2 | 17 [52]+ | |
| Araneae: Ctenidae | Amauropelma cf. sp. | TB | end | 4,9,12a | L | 24 [53] |
| Araneae: Psilodercidae | Psiloderces leclerci Deeleman-Rheinhold, 1995 | TB? | end | 2,4,13 | 38 [53] | |
| Araneae: Ochyroceratidae | Speocera caeca Deeleman-Rheinhold, 1995 | TB | end | 2,4,13 | L | 84 [53] |
| Araneae: Pholcidae | Spermophora maros Huber, 2005 | TB | end * | 2,9 | 45 [53] | |
| Araneae: Pholcidae | Uthina mimpi Huber, Caspar and Eberle, 2019 | TB | end | 2,15 | 17 [53] | |
| Opiliones | Opiliones sp. | TB | end | 2,9,12b,15 | n.a. | |
| Palpigradi: Eukoeneniidae | Eukoenenia maros Condé, 1992 | TB | end * | 9,13 | 93 [54] | |
| Palpigradi: Prokoeneniidae | Prokoenenia celebica Condé, 1994 | TB | end * | 2 | 6 [54] | |
| Pseudoscorpiones | Pseudoscorpiones sp. | TB | n.a. | 15 | L | n.a. |
| Schizomida | Schizomida spp. | TB | n.a. | 2,4,9,10a,15 | L | n.a. |
| Diplopoda: Haplodesmidae | Eutrichodesmus reductus Golovatch et al., 2009 | TB | end | 9 | L,S | 53 [51] |
| Diplopoda: Metopidiothrichidae | Metopidiothrix kalang Shear, 2002 | TB | end * | 2 | 39 [51] | |
| Oniscida: Philosciidae | Papuaphiloscia sp. | TB | end | 2,9,13 | L,S | 15 [51] |
| Oniscida: Armadillidae | Venezillo sp. | TB | end | 4,10a,12b,13,15 | S | 137 [51] |
| Collembola: Neanuridae | Deuterobella sp. | TB-TP? | end | 9 | L,S | 4 [55]+ |
| Collembola: Neelidae | Megalothorax sp. | TB-TP? | ? | 2 | L | 33 [55]+ |
| Collembola: Oncopoduridae | Oncopodura sp. | TB | end | 9 | L | 49 [55]+ |
| Collembola: Sminthuridae | Pararrhopalites sp. | TB | end? | 2,9,12b,15 | L | 17 [55]+ |
| Collembola: Entomobryidae | Pseudosinella maros Deharveng and Suhardjono, 2004 | TB | end | 2,4,5,9,12a,12b,15 | L,S | 352 [55]+ |
| Collembola: Entomobryidae | Sinella sp. | TB? | ? | 15 | 86 [55]+ | |
| Diplura: Campodeidae | Lepidocampa (Lepidocampa) hypogaea Condé, 1992 | TB | end | 2,4,6,9,12b,13 | 17 [56] | |
| Zygentoma: Nicoletiidae | Nicoletiidae | TB? | end | S | n.a. | |
| Blattodea: Nocticolidae | Nocticolidae sp. 1 | TB | end | 2?,9?,12a,12b?,15? | L?,S? | n.a. |
| Blattodea: Nocticolidae | Nocticolidae sp. 2 | TB | end | 2?,9,12a,12b?,15? | L,S? | n.a. |
| Coleoptera: Carabidae | Eustra saripaensis Deuve, 2002 | TB | end * | L | 28 [57] | |
| Coleoptera: Carabidae | Mateuellus troglobioticus troglobioticus Deuve, 1990 | TB | end | 6,15 | L,S | 2 [58] |
| Hemiptera: Cixiidae | Cixiidae sp. | TB? | ? | 15 | n.a. |
| Taxonomic Group | Taxon | Ecology | Dist | Towakkalak | Saripa | Gen Val |
|---|---|---|---|---|---|---|
| Caridea: Atyidae | Caridina parvidentata Roux, 1904 | SP | end | 9 | 344 [51] | |
| Caridea: Palaemonidae | Macrobrachium lar (Fabricius, 1798) | SP | pan | 9 | 276 [51] | |
| Brachyura: Gecarcinucidae | Parathelphusa celebensis (De Man, 1892) | SP | sul | Pa | 49 [51] | |
| Brachyura: Gecarcinucidae | Parathelphusa pareparensis (De Man, 1892) | SP | end | 2,9 | 49 [51] | |
| Pisces: Gobiidae | Glossogobius sp. | SP? | ? | 9 | 35 [51] | |
| Gastropoda: Subulinidae | Allopeas gracile (Hutton, 1834) | TP | pan | 15 | L | 21 [51] |
| Gastropoda: Subulinidae | Paropeas achatinaceum Pfeiffer, 1846 | TP | ipa | 15 | 4 [51] | |
| Amblypygi: Charontidae | Charon sp. | TP | ? | 10b,15 | L,S | 5 [52]+ |
| Araneae: Sparassidae | Heteropoda beroni Jaeger, 2005 | TP | end | 15 | L | 189 [53] |
| Araneae: Ochyroceratidae | Speocera karkari (Baert, 1980) | TP | ? | 13 | 84 [53] | |
| Diplopoda: Glomeridesmidae | Glomeridesmus sp. | TP? | ? | 15 | 28 [51] | |
| Diplopoda: Cambalopsidae | Hypocambala helleri Silvestri, 1897 | TP-TB | wid | 10b,15 | L | 15 [51] |
| Diplura: Campodeidae | Lepidocampa (L.) weberi borneensis Silvestri, 1933 | TP | wid | 2 | 17 [56] | |
| Collembola: Hypogastruridae | Acherontiella sp. | TP(eu) | ? | 15 | 20 [55]+ | |
| Collembola: Isotomidae | Isotomodes sp. | TP(eu) | ? | 15 | 36 [55]+ | |
| Collembola: Isotomidae | Folsomides centralis (Denis, 1931) | TP(Gu) | pan | 2,9 | 70 [55]+ | |
| Collembola: Isotomidae | Folsomides parvulus Stach, 1922 | TP(Gu) | cos | 2,5 | 70 [55]+ | |
| Collembola: Isotomidae | Folsomides pseudoparvulus Martynova, 1978 | TP(Gu) | pan | 2 | 70 [55]+ | |
| Collembola: Isotomidae | Folsomina onychiurina Denis, 1931 | TP(Gu) | pan | 2,5,12b | 5 [55]+ | |
| Collembola: Isotomidae | Isotomiella nummulifer Deharveng and Oliveira, 1990 | TP | pan | 2 | 55 [55]+ | |
| Collembola: Isotomidae | Isotomiella symetrimucronata Najt and Thibaud, 1988 | TP | pan | 2,15 | 55 [55]+ | |
| Collembola: Hypogastruridae | Willemia cf. buddenbrocki Hüther, 1959 | TP | ? | 2 | 47 [55]+ | |
| Collembola: Hypogastruridae | Xenylla yucatana Mills, 1938 | TP(Gu) | pan | 5 | 139 [55]+ | |
| Orthoptera: Rhaphidophoridae | Rhaphidophora sp. | TP | end | 2,9,12a,15 | L,S | 102 [59]+ |
| Coleoptera: Aderidae | Aderidae sp. | TP(Gu) | ? | 15 | L | n.a. |
| Coleoptera: Histeridae | Aeletes sp. | TP | ? | 2 | 87 [60] | |
| Coleoptera: Staphylinidae | Staphylinidae spp. | TP(Gu) | ? | 2,4,12a,12b,15 | L | n.a. |
| Lepidoptera: Tineidae | Tineidae spp. | TP(Gu) | ? | 5 | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deharveng, L.; Rahmadi, C.; Suhardjono, Y.R.; Bedos, A. The Towakkalak System, A Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia. Diversity 2021, 13, 392. https://doi.org/10.3390/d13080392
Deharveng L, Rahmadi C, Suhardjono YR, Bedos A. The Towakkalak System, A Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia. Diversity. 2021; 13(8):392. https://doi.org/10.3390/d13080392
Chicago/Turabian StyleDeharveng, Louis, Cahyo Rahmadi, Yayuk Rahayuningsih Suhardjono, and Anne Bedos. 2021. "The Towakkalak System, A Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia" Diversity 13, no. 8: 392. https://doi.org/10.3390/d13080392
APA StyleDeharveng, L., Rahmadi, C., Suhardjono, Y. R., & Bedos, A. (2021). The Towakkalak System, A Hotspot of Subterranean Biodiversity in Sulawesi, Indonesia. Diversity, 13(8), 392. https://doi.org/10.3390/d13080392