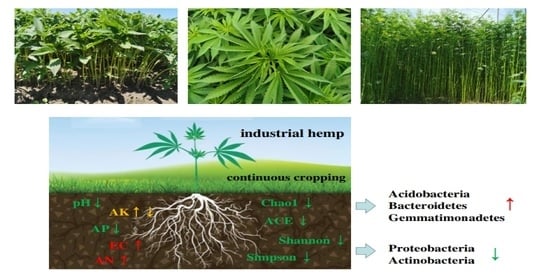
Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Sample Collection
2.3. Soil DNA Extraction and PCR Amplification of 16S rRNA
2.4. Illumina MiSeq Sequencing and Processing of Sequencing Date
2.5. Determination of Soil Physicochemical Properties
2.6. Data Analysis and Statistics
3. Results
3.1. Soil Physical and Chemical Properties of Industrial Hemp in Different Continuous Cropping Years
3.2. Effects of Different Continuous Cropping Years on Bacterial Diversity of Rhizosphere Soil
3.3. Effects of Different Continuous Cropping Years on the Bacterial Community of Rhizosphere Soil
3.4. Effect of Soil Factors on Bacterial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.; Fahad, S.; Du, G.; Cheng, X.; Yang, Y.; Tang, K.; Liu, L.; Liu, F.-H.; Deng, G. Evaluation of hemp (Cannabis sativa L.) as an industrial crop: A review. Environ. Sci. Pollut. Res. 2021, 28, 52832–52843. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, V.; Türkten, H.; Yıldırım, Ç.; Canan, S. Economic viability of industrial hemp production in Turkey. Ind. Crops Prod. 2022, 176, 114354. [Google Scholar] [CrossRef]
- Papstylianou, P.; Kakabouki, I.; Travlos, I. Effect of Nitrogen Fertilization on Growth and Yield of Industrial Hemp (Cannabis sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Duque Schumacher, A.G.; Pequito, S.; Pazour, J. Industrial hemp fiber: A sustainable and economical alternative to cotton. J. Clean. Prod. 2020, 268, 122180. [Google Scholar] [CrossRef]
- Parada-Rojas, C.H.; Pecota, K.; Almeyda, C.; Yencho, G.C.; Quesada-Ocampo, L.M. Sweetpotato Root Development Influences Susceptibility to Black Rot Caused by the Fungal Pathogen Ceratocystis fimbriata. Phytopathology 2021, 111, 1660–1669. [Google Scholar] [CrossRef]
- Han, G.; Lan, J.; Chen, Q.; Yu, C.; Bie, S. Response of soil microbial community to application of biochar in cotton soils with different continuous cropping years. Sci. Rep. 2017, 7, 10184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Zhou, Y.; He, H. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Li, Y.; Chi, J.; Ao, J.; Gao, X.; Liu, X.; Sun, Y.; Zhu, W. Effects of Different Continuous Cropping Years on Bacterial Community and Diversity of Cucumber Rhizosphere Soil in Solar-Greenhouse. Curr. Microbiol. 2021, 78, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, C.; Cheng, Z.; Meng, H.; Zhang, M.; Zhang, H. Soil Chemical Property Changes in Eggplant/Garlic Relay Intercropping Systems under Continuous Cropping. PLoS ONE 2014, 9, e111040. [Google Scholar] [CrossRef] [Green Version]
- Ramesha, G.K.; Leno, N.; Radhika, N.S. Linking root phenomics, nutrient acquisition and utilisation in amaranthus with thermochemical organic fertilizer from biowaste. Rhizosphere 2021, 20, 100426. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Gómez Expósito, R.; de Bruijn, I.; Postma, J.; Raaijmakers, J.M. Current Insights into the Role of Rhizosphere Bacteria in Disease Suppressive Soils. Front. Microbiol. 2017, 8, 2529. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, Z.; Zhou, J.; Guo, F.; Ding, Z.; Qiu, Z. Analysis of bacterial communities in rhizosphere soil of continuously cropped healthy and diseased konjac. World J. Microbiol. Biotechnol. 2017, 33, 134. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; JI, X. Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J. Basic Microbiol. 2017, 57, 337–344. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Y.; Han, M.; Xu, J.; Wang, X.; Liu, L.; Tang, Z.; Jiao, W.; Jin, R.; Liu, M.; et al. Effects of continuous cropping of sweet potatoes on the bacterial community structure in rhizospheric soil. BMC Microbiol. 2021, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, T.; Zheng, C.; Kang, C.; Yang, Z.; Yao, X.; Song, F.; Zhang, R.; Wang, X.; Xu, N.; et al. Changes in soil bacterial community structure as a result of incorporation of Brassica plants compared with continuous planting eggplant and chemical disinfection in greenhouses. PLoS ONE 2017, 12, e0173923. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-Y.; Li, H.; Hao, M.-M.; Ren, Y.-N.; Zhang, M.-K.; Liu, R.-Y.; Zhang, Y.; Li, G.; Chen, J.-S.; Ning, T.-Y.; et al. Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J. Appl. Ecol. 2021, 58, 2243–2255. [Google Scholar] [CrossRef]
- Jin, L.; Lyu, J.; Jin, N.; Xie, J.; Wu, Y.; Zhang, G.; Feng, Z.; Tang, Z.; Liu, Z.; Luo, S.; et al. Effects of different vegetable rotations on the rhizosphere bacterial community and tomato growth in a continuous tomato cropping substrate. PLoS ONE 2021, 16, e0257432. [Google Scholar] [CrossRef] [PubMed]
- Horne, M.; Mastrianni, K.R.; Amick, G.; Hardy, R.; Renneker, E.; Miller, K.W.P. Fast Discrimination of Marijuana using Automated High-throughput Cannabis Sample Preparation and Analysis by Gas Chromatography–Mass Spectrometry. J. Forensic Sci. 2020, 65, 1709–1715. [Google Scholar] [CrossRef]
- Small, E.; Brookes, B. Temperature and Moisture Content for Storage Maintenance of Germination Capacity of Seeds of Industrial Hemp, Marijuana, and Ditchweed Forms of Cannabis sativa. J. Nat. Fibers 2012, 9, 240–255. [Google Scholar] [CrossRef]
- Wu, Y.; Trejo, H.X.; Chen, G.; Li, S. Phytoremediation of contaminants of emerging concern from soil with industrial hemp (Cannabis sativa L.): A review. Environ. Dev. Sustain. 2021, 23, 14405–14435. [Google Scholar] [CrossRef]
- Tang, K.; Wang, J.; Yang, Y.; Deng, G.; Yu, J.; Hu, W.; Guo, L.; Du, G.; Liu, F. Fiber hemp (Cannabis sativa L.) yield and its response to fertilization and planting density in China. Ind. Crops Prod. 2022, 177, 114542. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Brodie, E.L.; DeSantis, T.Z.; Andersen, G.L.; Lindow, S.E.; Firestone, M.K. Selective progressive response of soil microbial community to wild oat roots. ISME J. 2009, 3, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Ashworth, A.J.; Owens, P.R.; Allen, F.L. Long-term cropping systems management influences soil strength and nutrient cycling. Geoderma 2020, 361, 114062. [Google Scholar] [CrossRef]
- Crookston, R.; Kurle, J.; Copeland, P.; Ford, J.; Lueschen, W. Rotational Cropping Sequence Affects Yield of Corn and Soybean. Agron. J. 1991, 83, 108–113. [Google Scholar] [CrossRef]
- Gentry, L.F.; Ruffo, M.L.; Below, F.E. Identifying Factors Controlling the Continuous Corn Yield Penalty. Agron. J. 2013, 105, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Fu, L.; Song, Y.; Yuan, L.; Zhang, H.; Wen, D.; Yang, N.; Wang, X.; Yue, Y.; Li, X.; et al. Effects of continuous cucumber cropping on crop quality and soil fungal community. Environ. Monit. Assess. 2021, 193, 436. [Google Scholar] [CrossRef]
- Alami, M.M.; Xue, J.; Ma, Y.; Zhu, D.; Abbas, A.; Gong, Z.; Wang, X. Structure, Function, Diversity, and Composition of Fungal Communities in Rhizospheric Soil of Coptis chinensis Franch under a Successive Cropping System. Plants 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil Metagenomics Reveals Effects of Continuous Sugarcane Cropping on the Structure and Functional Pathway of Rhizospheric Microbial Community. Front. Microbiol. 2021, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, Q.; Xu, S.; Ma, L.; Tian, C. Long-term effect of residue return and fertilization on microbial biomass and community composition of a clay loam soil. J. Agric. Sci. 2016, 154, 1051–1061. [Google Scholar] [CrossRef]
- Zu, C.; Li, Z.; Yang, J.; Yu, H.; Sun, Y.; Tang, H.; Yost, R.; Wu, H. Acid Soil Is Associated with Reduced Yield, Root Growth and Nutrient Uptake in Black Pepper ( Piper nigrum L.). J. Agric. Sci. 2014, 5, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Tang, D.; Mao, L.; Zhang, D.; Zhou, P.; Zhi, Y.; Zhang, J. Phytoremediation of secondary saline soil by halophytes with the enhancement of γ-polyglutamic acid. Chemosphere 2021, 285, 131450. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, Y.; Gong, J. Physiological mechanisms of the tolerance response to manganese stress exhibited by Pinus massoniana, a candidate plant for the phytoremediation of Mn-contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 45422–45433. [Google Scholar] [CrossRef]
- Wallenstein, M.D. Managing and manipulating the rhizosphere microbiome for plant health: A systems approach. Rhizosphere 2017, 3, 230–232. [Google Scholar] [CrossRef]
- Hu, J.; Masson, R. First Report of Crown and Root Rot Caused by Pythium aphanidermatum on Industrial Hemp (Cannabis sativa) in Arizona. Plant Dis. 2021, 105, 2257. [Google Scholar] [CrossRef]
- Sikorski, J.; Baumgartner, V.; Birkhofer, K.; Boeddinghaus, R.S.; Bunk, B.; Fischer, M.; Fösel, B.U.; Friedrich, M.W.; Göker, M.; Hölzel, N.; et al. The Evolution of Ecological Diversity in Acidobacteria. Front. Microbiol. 2022, 13, 715637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Chen, S.; Qi, G.; He, Z.; Zhao, X. Microbial taxa and functional genes shift in degraded soil with bacterial wilt. Sci. Rep. 2017, 7, 39911. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, S.; Jiang, Y.; Lin, Z.; Luo, J.; Li, M.; Guo, J.; Su, Y.; Xu, L.; Que, Y. The Physiological and Agronomic Responses to Nitrogen Dosage in Different Sugarcane Varieties. Front. Plant Sci. 2019, 10, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]







| Type | pH | EC (ms/cm) | SOM (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|---|
| R1 | 8.52 ± 0.03 a | 189.15 ± 6.45 c | 26.16 ± 0.38 a | 134.46 ± 0.63 b | 14.53 ± 0.79 a | 154.90 ± 26.12 b |
| R2 | 8.40 ± 0.01 b | 190.90 ± 6.50 c | 26.80 ± 0.75 a | 170.68 ± 1.95 a | 13.92 ± 0.91 ab | 210.43 ± 4.12 ab |
| R3 | 8.38 ± 0.01 b | 195.15 ± 8.85 bc | 26.67 ± 0.59 a | 162.60 ± 25.50 ab | 12.71 ± 1.32 b | 285.25 ± 93.66 a |
| R4 | 8.15 ± 0.03 c | 205.00 ± 3.00 ab | 25.72 ± 0.72 a | 150.57 ± 22.75 ab | 10.83 ± 0.18 c | 280.21 ± 50.73 a |
| R5 | 7.78 ± 0.06 d | 209.00 ± 7.00 a | 25.91 ± 0.41 a | 179.84 ± 3.79 a | 10.78 ± 1.09 c | 186.74 ± 39.77 ab |
| Type | Ace Index | Chao1 Index | Shannon Index | Simpson Index | Coverage Index |
|---|---|---|---|---|---|
| R1 | 1887.22 ± 20.29 a | 1903.85 ± 21.84 a | 6.04 ± 0.02 a | 0.99 ± 0.008 a | 0.99 ± 0.00100 a |
| R2 | 1821.16 ± 16.63 b | 1822.37 ± 23.46 b | 6.04 ± 0.02 a | 0.96 ± 0.005 b | 0.99 ± 0.00010 a |
| R3 | 1770.48 ± 19.23 c | 1759.64 ± 27.78 c | 5.68 ± 0.04 b | 0.93 ± 0.008 c | 0.99 ± 0.00004 a |
| R4 | 1637.78 ± 4.78 d | 1624.82 ± 10.57 d | 5.26 ± 0.07 c | 0.87 ± 0.007 d | 0.99 ± 0.00045 a |
| R5 | 1521.22 ± 9.51 e | 1520.01 ± 11.67 e | 5.05 ± 0.02 d | 0.83 ± 0.005 e | 0.99 ± 0.00030 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Chen, X.; Li, Z.; Wang, M.; Che, Y.; Zhang, L.; Jiang, Z.; Jie, S. Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment. Diversity 2022, 14, 250. https://doi.org/10.3390/d14040250
Guo L, Chen X, Li Z, Wang M, Che Y, Zhang L, Jiang Z, Jie S. Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment. Diversity. 2022; 14(4):250. https://doi.org/10.3390/d14040250
Chicago/Turabian StyleGuo, Li, Xiangwei Chen, Zeyu Li, Mingze Wang, Ye Che, Ling Zhang, Zeyu Jiang, and Siyuan Jie. 2022. "Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment" Diversity 14, no. 4: 250. https://doi.org/10.3390/d14040250
APA StyleGuo, L., Chen, X., Li, Z., Wang, M., Che, Y., Zhang, L., Jiang, Z., & Jie, S. (2022). Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment. Diversity, 14(4), 250. https://doi.org/10.3390/d14040250