Backyard Biomes: Is Anyone There? Improving Public Awareness of Urban Wildlife Activity
Abstract
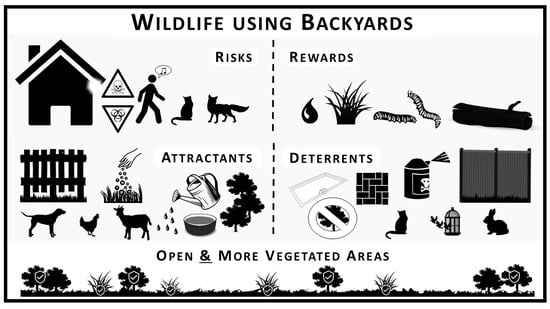
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Household Activity Surveys
2.3. Backyard Animal Activity Surveys
2.4. Statistical Analyses
3. Results
3.1. Household Activity Surveys
3.2. Backyard Animal Activity Surveys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, R.I.; Kareiva, P.; Forman, R.T. The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol. Conserv. 2008, 141, 1695–1703. [Google Scholar] [CrossRef]
- McRae, B.H.; Schumaker, N.H.; McKane, R.B.; Busing, R.T.; Solomon, A.M.; Burdick, C.A. A multi-model framework for simulating wildlife population response to land-use and climate change. Ecol. Model. 2008, 219, 77–91. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C. Novel Ecosystems: Intervening in the New Ecological World Order; John Wiley and Sons: West Sussex, UK, 2013. [Google Scholar]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Weller, R.J.; Hoch, C.; Huang, C. Atlas for the End of the World. 2017. Available online: http://atlas-for-the-end-of-the-world.com (accessed on 16 November 2021).
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.; et al. Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Cresswell, I.D.; Murphy, H. Biodiversity. In Australia State of the Environment 2016; Australian Government Department of the Environment and Energy: Canberra, Australia, 2016. [Google Scholar] [CrossRef]
- Van der Ree, R. The impact of urbanisation on the mammals of Melbourne—Do atlas records tell the whole story or just some of the chapters. In Urban Wildlife: More than Meets the Eye; Lunney, D., Burgin, S., Eds.; Royal Zoological Society of New South Wales: Mosman, NSW, Australia, 2004; pp. 195–204. [Google Scholar]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Cox, D.T.; Gaston, K.J. Human—nature interactions and the consequences and drivers of provisioning wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170092. [Google Scholar] [CrossRef] [Green Version]
- Parsons, A.W.; Forrester, T.; Baker-Whatton, M.C.; McShea, W.J.; Rota, C.T.; Schuttler, S.G.; Millspaugh, J.J.; Kays, R. Mammal communities are larger and more diverse in moderately developed areas. eLife 2018, 7, e38012. [Google Scholar] [CrossRef] [PubMed]
- Berger, J. Fear, human shields and the redistribution of prey and predators in protected areas. Biol. Lett. 2007, 3, 620–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, J.D.; Cleeton, S.H.; Lyons, T.P.; Miller, J.R. Urbanization and the predation paradox: The role of trophic dynamics in structuring vertebrate communities. Bioscience 2012, 62, 809–818. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Kearns, L.J.; Shustack, D.P. Anthropogenic resource subsidies decouple predator-prey relationships. Ecol. Appl. 2011, 21, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.; Polderman, A.; Birkenholtz, T. Lawns and toxins: An ecology of the city. Cities 2001, 18, 369–380. [Google Scholar] [CrossRef]
- Doherty, T.S.; Dickman, C.R.; Glen, A.S.; Newsome, T.M.; Nimmo, D.G.; Ritchie, E.G.; Vanak, A.T.; Wirsing, A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Conserv. 2017, 210, 56–59. [Google Scholar] [CrossRef]
- Legge, S.; Woinarski, J.C.; Dickman, C.R.; Murphy, B.P.; Woolley, L.A.; Calver, M.C. We need to worry about Bella and Charlie: The impacts of pet cats on Australian wildlife. Wildl. Res. 2020, 47, 523–539. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Halfwerk, W.; Baird, E.; Buxton, R.T.; Fernández-Juricic, E.; Fristrup, K.M.; McKenna, M.F.; Mennitt, D.J.; Perkin, E.K.; Seymoure, B.M.; et al. Why conservation biology can benefit from sensory ecology. Nat. Ecol. Evol. 2020, 4, 502–511. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Gaston, K.J.; Ávila-Jiménez, M.L.; Edmondson, J.L.; Jones, J. Managing urban ecosystems for goods and services. J. Appl. Ecol. 2013, 50, 830–840. [Google Scholar] [CrossRef]
- Lerman, S.B.; Narango, D.L.; Andrade, R.; Warren, P.S.; Grade, A.M.; Straley, K. Wildlife in the city: Human drivers and human consequences. In Urban Ecology: It’s Nature and Challenges; Barbosa, P., Ed.; CABI Publishing: Boston, MA, USA, 2021; pp. 37–66. [Google Scholar]
- Laundré, J.W.; Hernández, L.; Altendorf, K.B. Wolves, elk, and bison: Re-establishing the “landscape of fear” in Yellowstone National Park, USA. Can. J. Zool. 2001, 79, 1401–1409. [Google Scholar] [CrossRef]
- Fardell, L.L.; Nano, C.E.M.; Pavey, C.R.; Dickman, C.R. Small prey animal habitat use in landscapes of fear: Effects of predator presence and human activity along an urban disturbance gradient. Front. Ecol. Evol. 2021, 9, 750094. [Google Scholar] [CrossRef]
- Fardell, L.L.; Nano, C.E.M.; Pavey, C.R.; Dickman, C.R. Small prey animal foraging behaviours in landscapes of fear: Effects of predator presence and human activity along an urban disturbance gradient. Front. Ecol. Evol. 2022, 10, 805891. [Google Scholar] [CrossRef]
- Wheatley, R.; Pavlic, T.P.; Levy, O.; Wilson, R.S. Habitat features and performance interact to determine the outcomes of terrestrial predator–prey pursuits. J. Anim. Ecol. 2020, 89, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Clinchy, M.; Zanette, L.Y.; Roberts, D.; Suraci, J.P.; Buesching, C.D.; Newman, C.; Macdonald, D.W. Fear of the human “super predator” far exceeds the fear of large carnivores in a model mesocarnivore. Behav. Ecol. 2016, 27, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Makin, D.F.; Chamaillé-Jammes, S.; Shrader, A.M. Herbivores employ a suite of antipredator behaviours to minimize risk from ambush and cursorial predators. Anim. Behav. 2017, 127, 225–231. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Wang, H.; Feng, J. Arms race of temporal partitioning between carnivorous and herbivorous mammals. Sci. Rep. 2018, 8, 1713. [Google Scholar] [CrossRef] [Green Version]
- Aplin, K.P.; Chesser, T.; ten Have, J. Evolutionary biology of the genus Rattus: Profile of an archetypal rodent pest. In Rats, Mice and People: Rodent Biology and Management; Singleton, G.R., Hinds, L.A., Krebs, C.J., Spratt, D.M., Eds.; ACIAR: Canberra, Australia, 2003; pp. 487–498. [Google Scholar]
- Feng, A.Y.; Himsworth, C.G. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosyst. 2014, 17, 149–162. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience 2017, 67, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, P.R.; Kennedy, D. Millennium assessment of human behaviour. Science 2005, 309, 562–563. [Google Scholar] [CrossRef]
- Ban, N.C.; Mills, M.; Tam, J.; Hicks, C.C.; Klain, S.; Stoeckl, N.; Bottrill, M.C.; Levine, J.; Pressey, R.L.; Satterfield, T.; et al. A social–ecological approach to conservation planning: Embedding social considerations. Front. Ecol. Environ. 2013, 11, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Why garden for wildlife? Social and ecological drivers, motivations and barriers for biodiversity management in residential landscapes. Ecol. Econ. 2013, 86, 258–273. [Google Scholar] [CrossRef]
- Balmford, A.; Cowling, R.M. Fusion or failure? The future of conservation biology. Conserv. Biol. 2006, 20, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R. Biodiversity conservation and the extinction of experience. Trends Ecol. Evol. 2005, 20, 430–434. [Google Scholar] [CrossRef]
- Chawla, L.; Cushing, D.F. Education for strategic environmental behaviour. Environ. Educ. Res. 2007, 13, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Dahmus, M.E.; Nelson, K.C. Yard stories: Examining residents’ conceptions of their yards as part of the urban ecosystem in Minnesota. Urban Ecosyst. 2014, 17, 173–194. [Google Scholar] [CrossRef]
- Osbaldiston, R.; Schott, J.P. Environmental sustainability and behavioural science. Environ. Behav. 2012, 44, 257–299. [Google Scholar] [CrossRef]
- Byerly, H.; Balmford, A.; Ferraro, P.J.; Hammond Wagner, C.; Palchak, E.; Polasky, S.; Ricketts, T.H.; Schwartz, A.J.; Fisher, B. Nudging pro-environmental behaviour: Evidence and opportunities. Front. Ecol. Environ. 2018, 16, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Cosquer, A.; Raymond, R.; Prévot-Julliard, A.-C. Observations of everyday biodiversity: A new perspective for conservation? Ecol. Soc. 2012, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Prévot, A.-C.; Cheval, H.; Raymond, R.; Cosquer, A. Routine experiences of nature in cities can increase personal commitment toward biodiversity conservation. Biol. Conserv. 2018, 226, 1–8. [Google Scholar] [CrossRef]
- Deguines, N.; Princé, K.; Prévot, A.C.; Fontaine, B. Assessing the emergence of pro-biodiversity practices in citizen scientists of a backyard butterfly survey. Sci. Total Environ. 2020, 716, 136842. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.C. Towards a coherent theory of environmentally significant behaviour. J. Soc. Issues 2000, 56, 407–424. [Google Scholar] [CrossRef]
- Clayton, S. Domesticated nature: Motivations for gardening and perceptions of environmental impact. J. Environ. Psychol. 2007, 27, 215–224. [Google Scholar] [CrossRef]
- Barrault, J. Gardening Practices Facing the Health and Environmental Risks of Pesticides: Differentiated Approaches in France and Quebec. Ph.D. Thesis, Université Toulouse le Mirail-Toulouse II, Toulouse, France, 2012. Available online: https://tel.archives-ouvertes.fr/tel-00859540 (accessed on 21 November 2021).
- Magle, S.B.; Hunt, V.M.; Vernon, M.; Crooks, K.R. Urban wildlife research: Past, present, and future. Biol. Conserv. 2012, 155, 23–32. [Google Scholar] [CrossRef]
- Collins, M.K.; Magle, S.B.; Gallo, T. Global trends in urban wildlife ecology and conservation. Biol. Conserv. 2021, 261, 109236. [Google Scholar] [CrossRef]
- Soanes, K.; Lentini, P.E. When cities are the last chance for saving species. Front. Ecol. Environ. 2019, 17, 225–231. [Google Scholar] [CrossRef]
- Department of Environment, Climate Change and Water (DECCW). Glenrock State Conservation Area Plan of Management; DECCW: Sydney, NSW, Australia, 2010.
- Bell, S.A.J. Volume 2: Vegetation Community Profiles, Lake Macquarie Local Government Area, East Coast Flora Survey, Working Draft v2. Unpublished Report to Lake Macquarie City Council. 2016. Available online: https://www.researchgate.net/publication/299749214_Volume_2_Vegetation_Community_Profiles_Lake_Macquarie_Local_Government_Area_Working_Draft_v2 (accessed on 5 October 2021).
- Fardell, L.L.; Young, L.I.; Pavey, C.R.; Dickman, C.R. Habitat use by wandering pet cats (Felis catus) in a patchy urban environment. J. Urban Ecol. 2021, 7, juab019. [Google Scholar] [CrossRef]
- Fleming, P.; Meek, P.; Ballard, G.; Banks, P.; Calridge, A.; Sanderson, J.; Swann, D. Camera Trapping: Wildlife Management and Research; CSIRO Publishing: Clayton, Australia, 2014. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package, Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 October 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package, Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 October 2021).
- Wei, T.; Simko, V. R Package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 16 November 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- McFadden, D. Quantitative Methods for Analysing Travel Behaviour of Individuals: Some Recent Developments. CFDP 1977, 707. Available online: https://elischolar.library.yale.edu/cgi/viewcontent.cgi?article=1706&context=cowles-discussion-paper-series (accessed on 5 October 2021).
- Jakes, A.F.; Jones, P.F.; Paige, L.C.; Seidler, R.G.; Huijser, M.P. A fence runs through it: A call for greater attention to the influence of fences on wildlife and ecosystems. Biol. Conserv. 2018, 227, 310–318. [Google Scholar] [CrossRef]
- Kays, R.; Parsons, A.W. Mammals in and around suburban yards, and the attraction of chicken coops. Urban Ecosyst. 2014, 17, 691–705. [Google Scholar] [CrossRef]
- Hansen, C.P.; Parsons, A.W.; Kays, R.; Millspaugh, J.J. Does use of backyard resources explain the abundance of urban wildlife? Front. Ecol. Evol. 2020, 8, 374. [Google Scholar] [CrossRef]
- Cook, E.M.; Hall, S.J.; Larson, K.L. Residential landscapes as social-ecological systems: A synthesis of multi-scalar interactions between people and their home environment. Urban Ecosyst. 2012, 15, 19–52. [Google Scholar] [CrossRef]
- Byrne, L.B.; Bruns, M.A. The effects of lawn management on soil microarthropods. J. Agric. Urban Entomol. 2004, 21, 150–156. [Google Scholar]
- Cheng, Z.; Grewal, P.S.; Stinner, B.R.; Hurto, K.A.; Hamza, H.B. Effects of long-term turfgrass management practices on soil nematode community and nutrient pools. Appl. Soil Ecol. 2008, 38, 174–184. [Google Scholar] [CrossRef]
- Muratet, A.; Fontaine, B. Contrasting impacts of pesticides on butterflies and bumblebees in private gardens in France. Biol. Conserv. 2015, 182, 148–154. [Google Scholar] [CrossRef]
- Bertoncini, A.P.; Machon, N.; Pavoine, S.; Muratet, A. Local gardening practices shape urban lawn floristic communities. Landsc. Urban Plan. 2012, 105, 53–61. [Google Scholar] [CrossRef]
- Ramesh, T.; Downs, C.T. Impact of land use on occupancy and abundance of terrestrial mammals in the Drakensberg Midlands, South Africa. J. Nat. Conserv. 2015, 23, 9–18. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Suckling, D.M.; Kimberley, M.; Richardson, B.; Coker, G.; Gous, S.; Kerr, J.L.; Cowan, D.M.; Lance, D.R.; Strand, T.; et al. Aerial application of pheromones for mating disruption of an invasive moth as a potential eradication tool. PLoS ONE 2012, 7, e43767. [Google Scholar] [CrossRef] [Green Version]
- Daane, K.M.; Almeida, R.P.; Bell, V.A.; Walker, J.T.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M.; et al. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 271–307. [Google Scholar]
- Sandberg, L.A.; Foster, J. Challenging lawn and order: Environmental discourse and lawn care reform in Canada. Environ. Polit. 2005, 14, 478–494. [Google Scholar] [CrossRef]
- Sharma, G.; Malthankar, P.A.; Mathur, V. Insect–Plant Interactions: A Multilayered Relationship. Ann. Entomol. Soc. Am. 2021, 114, 1–16. [Google Scholar] [CrossRef]
- Jones, M.E.; Apfelbach, R.; Banks, P.B.; Cameron, E.Z.; Dickman, C.R.; Frank, A.; McLean, S.; McGregor, I.S.; Müller-Schwarze, D.; Parsons, M.H.; et al. A nose for death: Integrating trophic and informational networks for conservation and management. Front. Ecol Evol. 2016, 4, 124. [Google Scholar] [CrossRef] [Green Version]
- Shannon, G.; McKenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J.; et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2016, 91, 982–1005. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, L.M.; Hames, F.; Faulkner, R.; Geschke, A.; Squires, Z.E.; McLeod, E.M. Putting the cat before the wildlife: Exploring cat owners’ beliefs about cat containment as predictors of owner behavior. Conserv. Sci. Pract. 2021, 3, e502. [Google Scholar] [CrossRef]
- Willson, S.K.; Okunlola, I.A.; Novak, J.A. Birds be safe: Can a novel cat collar reduce avian mortality by domestic cats (Felis catus)? Glob. Ecol. Conserv. 2015, 3, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Gompper, M.E. Adding Nuance to Our Understanding of Dog–Wildlife Interactions and the Need for Management. Integr. Comp. Biol. 2021, 61, 93–102. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Heppner, F. Sensory mechanisms and environmental clues used by the American Robin in locating earthworms. Condor 1965, 67, 247–256. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman and Hall: London, UK, 1996; Volume 3. [Google Scholar]
- Lunney, D.; Burgin, S. Urban wildlife management: Forming an Australian synthesis. In Urban Wildlife: More than Meets the Eye; Lunney, D., Burgin, S., Eds.; Royal Zoological Society of New South Wales: Mosman, NSW, Australia, 2004; pp. 230–247. [Google Scholar]
- Daniels, G.D.; Kirkpatrick, J.B. Does variation in garden characteristics influence the conservation of birds in suburbia? Biol. Conserv. 2006, 133, 326–335. [Google Scholar] [CrossRef]
- Magle, S.B.; Theobald, D.M.; Crooks, K.R. A comparison of metrics predicting landscape connectivity for a highly interactive species along an urban gradient in Colorado, USA. Landsc. Ecol. 2009, 24, 267–280. [Google Scholar] [CrossRef]
- Hartig, T.; Evans, G.W.; Jamner, L.D.; Davis, D.S.; Gärling, T. Tracking restoration in natural and urban field settings. J. Environ. Psychol. 2003, 23, 109–123. [Google Scholar] [CrossRef]
- Tzoulas, K.; Korpela, K.; Venn, S.; Yli-Pelkonen, V.; Kaźmierczak, A.; Niemela, J.; James, P. Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landsc. Urban Plan. 2007, 81, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, A.E.; Maas, J.; Verheij, R.A.; Groenewegen, P.P. Green space as a buffer between stressful life events and health. Soc. Sci. Med. 2010, 70, 1203–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.J.; Akbari, H.; Taha, H. The wind-shielding and shading effects of trees on residential heating and cooling requirements. ASHRAE Proc. 1990, 96, 1403–1411. [Google Scholar]
- Akbari, H.; Kurn, D.M.; Bretz, S.E.; Hanford, J.W. Peak power and cooling energy savings of shade trees. Energy Build. 1997, 25, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.R. Improved estimates of tree-shade effects on residential energy use. Energy Build. 2002, 34, 1067–1076. [Google Scholar] [CrossRef]
- Khachatryan, H.; Rihn, A.; Hansen, G.; Clem, T. Landscape aesthetics and maintenance perceptions: Assessing the relationship between homeowners’ visual attention and landscape care knowledge. Land Use Policy 2020, 95, 104645. [Google Scholar] [CrossRef]
- Wat, K.K.; Banks, P.B.; McArthur, C. Linking animal personality to problem-solving performance in urban common brushtail possums. Anim. Behav. 2020, 162, 35–45. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization, Biodiversity, and Conservation. The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Head, L.; Muir, P. Edges of connection: Reconceptualising the human role in urban biogeograpy. Aust. Geogr. 2006, 37, 87–101. [Google Scholar] [CrossRef]



| Pets | |||||||||
| none | dog | cat | bird | chickens | goat | rabbit | |||
| 26 | 17 | 11 | 7 | 6 | 1 | 1 | |||
| Garden type | |||||||||
| semi-natural | modified-natural | semi-modified | modified | very modified | |||||
| 1 | 1 | 17 | 19 | 12 | |||||
| Watering frequency | |||||||||
| never | bimonthly | monthly | fortnightly | once a week | twice a week | second daily | daily | ||
| 4 | 16 | 3 | 4 | 10 | 7 | 2 | 4 | ||
| Continual water source available | |||||||||
| yes | no | ||||||||
| 36 | 14 | ||||||||
| Fertiliser frequency | |||||||||
| never | yearly | 6 monthly | 4 monthly | 3 monthly | 2 monthly | monthly | |||
| 17 | 8 | 9 | 8 | 6 | 1 | 1 | |||
| A combination of fertiliser types was used | |||||||||
| compost | fish emulsion/seaweed solution | Manure | blood and bone | wetting agent | targeted fertiliser (plant/lawn/fruit/flower) | dynamic lifter | weed and feed | ||
| 4 | 5 | 8 | 1 | 1 | 16 | 9 | 2 | ||
| Pesticide frequency | |||||||||
| never | 2 yearly | yearly | 6 monthly | 4 monthly | 3 monthly | 2 monthly | monthly | ||
| 23 | 3 | 9 | 7 | 5 | 2 | 1 | 1 | ||
| A combination of pesticide types was used | |||||||||
| ant powder/spray | cockroach spray/bait | spider spray | professional multi pesticides | insecticide (pyrethrum/baythroid/bifenthrin) | fruit tree fungicide | lawn weeder (bindi/clover, roundup, weed and feed) | natural pesticide spray | home remedies (white oil, hot water, vinegar, garlic, chilli) | |
| 4 | 5 | 2 | 4 | 5 | 2 | 9 | 2 | 9 | |
| Light in yard frequency | |||||||||
| never | rarely | monthly | weekly | sensor | all night | 6 h night | 3 h night | 1 h night | |
| 22 | 5 | 1 | 2 | 15 | 1 | 2 | 1 | 1 | |
| Light in yard watts | |||||||||
| 0 W | 40 W | 50 W | 60 W | 100 W | 150 W | 200 W | |||
| 22 | 1 | 7 | 12 | 4 | 1 | 3 | |||
| Appliance/people noise frequency | |||||||||
| never | rarely | fortnightly | weekly | daily | afternoons | evenings | |||
| 20 | 3 | 2 | 1 | 14 | 1 | 8 | |||
| Moderate music frequency | |||||||||
| never | 3 monthly | weekly | daily | mornings | evenings | ||||
| 34 | 1 | 2 | 9 | 2 | 2 | ||||
| Fence type | |||||||||
| open gap | full gap | open ground | full ground | ||||||
| 14 | 11 | 4 | 21 | ||||||
| Primary activity in surrounding conservation area and connected green spaces | |||||||||
| none | walk | run | cycle | dog walk | horse ride | mowing | |||
| 4 | 33 | 4 | 2 | 5 | 1 | 1 | |||
| Frequency of this activity | |||||||||
| none | rarely | 3 monthly | monthly | weekly | twice a week | five times a week | daily | ||
| 4 | 1 | 1 | 9 | 19 | 4 | 1 | 11 | ||
| Secondary activities in surrounding conservation area and connected green spaces (multiple for some) | |||||||||
| none | walk | cycle | dog walk | ||||||
| 31 | 7 | 13 | 2 | ||||||
| Frequency of these activities | |||||||||
| none | 6 monthly | 4 monthly | monthly | weekly | |||||
| 31 | 1 | 1 | 11 | 6 | |||||
| Variable | ANOSIM R | Significance |
|---|---|---|
| Yard Light Watts | 0.07164 | 0.1473 |
| Light Used in Yard (y/n) | −0.02777 | 0.8474 |
| Light in Yard Frequency | 0.06116 | 0.1553 |
| Water Source Available (y/n) | −0.09947 | 0.9712 |
| Watering Frequency | −0.04416 | 0.7341 |
| Garden Type | −0.04067 | 0.8452 |
| Fertiliser Used (y/n) | −0.05675 | 0.8682 |
| Fertiliser Frequency | −0.0189 | 0.5983 |
| Pesticide Used (y/n) | 0.05445 | 0.0514 |
| Pesticide Frequency | −0.01336 | 0.5439 |
| Fence Type | 0.08198 | 0.0586 |
| Noise in Yard (y/n) | −0.105 | 0.9609 |
| Noise Frequency | 0.1588 | 0.0118 |
| Pet in Yard (y/n) | 0.07224 | 0.0342 |
| Pet in Yard Frequency | 0.1686 | 0.0148 |
| Number of Cats Present | 0.01956 | 0.3808 |
| Number of Dogs Present | 0.1019 | 0.0812 |
| Response Variable | Explanatory Variable | Estimate | Standard Error | Test Statistic | P-Value | Confidence Interval (Low) | Confidence Interval (High) |
|---|---|---|---|---|---|---|---|
| antechinus | (Intercept) | 1.01 | 0.24 | 0.05 | 0.96 | 0.63 | 1.62 |
| antechinus | Area (open) | 0.33 | 0.50 | −2.24 | 0.03 | 0.12 | 0.87 |
| antechinus | Period (day) | 0.45 | 0.84 | −0.96 | 0.34 | 0.09 | 2.31 |
| bandicoot | (Intercept) | 0.33 | 0.35 | −3.20 | 0.00 | 0.17 | 0.65 |
| bandicoot | Area (open) | 0.49 | 0.71 | −1.01 | 0.31 | 0.12 | 1.95 |
| bandicoot | Period (day) | 0.00 | 0.00 | −1.96 × 1014 | 0.00 | 0.00 | 0.00 |
| brushtail | (Intercept) | 1.65 | 0.22 | 2.30 | 0.02 | 1.08 | 2.52 |
| brushtail | Area (open) | 1.30 | 0.35 | 0.76 | 0.45 | 0.66 | 2.56 |
| brushtail | Period (day) | 0.00 | 0.00 | −6.59 × 1015 | 0.00 | 0.00 | 0.00 |
| cat | (Intercept) | 0.26 | 0.38 | −3.59 | 0.00 | 0.12 | 0.54 |
| cat | Area (open) | 0.90 | 0.63 | −0.17 | 0.87 | 0.26 | 3.08 |
| cat | Period (day) | 0.52 | 1.12 | −0.59 | 0.56 | 0.06 | 4.66 |
| dog | (Intercept) | 0.21 | 0.37 | −4.23 | 0.00 | 0.10 | 0.43 |
| dog | Area (open) | 2.38 | 0.46 | 1.88 | 0.06 | 0.97 | 5.87 |
| dog | Period (day) | 18.62 | 0.49 | 6.02 | 0.00 | 7.18 | 48.27 |
| fox | (Intercept) | 0.00 | 0.51 | −242.65 | 0.00 | 0.00 | 0.00 |
| fox | Area (open) | 5.38 × 1052 | 0.51 | 236.94 | 0.00 | 1.97 × 1052 | 1.47 × 1053 |
| fox | Period (day) | 0.00 | 56.45 | −0.12 | 0.90 | 0.00 | 1.22 × 1045 |
| native bird | (Intercept) | 0.04 | 0.75 | −4.31 | 0.00 | 0.01 | 0.17 |
| native bird | Area (open) | 0.89 | 0.58 | −0.19 | 0.85 | 0.29 | 2.78 |
| native bird | Period (day) | 100.95 | 0.84 | 5.47 | 0.00 | 19.30 | 527.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardell, L.L.; Pavey, C.R.; Dickman, C.R. Backyard Biomes: Is Anyone There? Improving Public Awareness of Urban Wildlife Activity. Diversity 2022, 14, 263. https://doi.org/10.3390/d14040263
Fardell LL, Pavey CR, Dickman CR. Backyard Biomes: Is Anyone There? Improving Public Awareness of Urban Wildlife Activity. Diversity. 2022; 14(4):263. https://doi.org/10.3390/d14040263
Chicago/Turabian StyleFardell, Loren L., Chris R. Pavey, and Chris R. Dickman. 2022. "Backyard Biomes: Is Anyone There? Improving Public Awareness of Urban Wildlife Activity" Diversity 14, no. 4: 263. https://doi.org/10.3390/d14040263
APA StyleFardell, L. L., Pavey, C. R., & Dickman, C. R. (2022). Backyard Biomes: Is Anyone There? Improving Public Awareness of Urban Wildlife Activity. Diversity, 14(4), 263. https://doi.org/10.3390/d14040263