Microalgae Indicators of Charophyte Habitats of South and Southeast Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Sampling and Laboratory Study
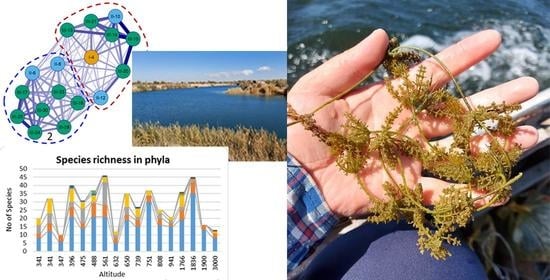
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domozych, D.; Popper, Z.A.; Sorensen, I. Charophytes: Evolutionary giants and emerging model organisms. Front. Plant Sci. 2016, 7, 1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palamar-Mordvintseva, G.M.; Tsarenko, P.M.; Barinova, S. Phylogenesis, Origin and Kinship of the Charophytic Algae. Bot. Pac. 2015, 4, 59–70. [Google Scholar] [CrossRef]
- Shepherd, V.; Beilby, M.; Heslop, D. Ecophysiology of the hypotonic response in the salt-tolerant charophyte alga Lamprothamnium papulosum. Plant Cell Environ. 1999, 22, 333–346. [Google Scholar] [CrossRef]
- Pełechata, A.; Pełechaty, M. The in-situ influence of Ceratophyllum demersum on a phytoplankton assemblage. Oceanol. Hydrobiol. Stud. 2010, 39, 95–101. [Google Scholar] [CrossRef]
- Wade, P. The colonization of disturbed freshwater habitats by Characeae. Folia Geobot. Phytotaxon. 1990, 25, 275–278. [Google Scholar] [CrossRef]
- Khuram, I.; Ahmad, N.; Barinova, S. Effect of water quality on the spatial distribution of charophytes in the Peshawar Valley, Khyber Pakhtunkhwa, Pakistan. Oceanol. Hydrobiol. Stud. 2021, 50, 359–372. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R.; Solak, C.N. New record of Chara hispida (L.) Hartm. (Streptophyta: Charophyceae, Charales) from the Işıklı Lake (Turkey) and critical checklist of Turkish charophytes. Nat. Resour. Conserv. 2014, 2, 33–42. [Google Scholar] [CrossRef]
- Schneider, S.C.; Garcia, A.; Martin-Closas, C.; Chivas, A.R. The role of charophytes (Charales) in past and present environments: An overview. Aquat. Bot. 2015, 120, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Stelzer, D.; Schneider, S.; Melzer, A. Macrophyte-based assessment of lakes—A contribution to the implementation of the European Water Framework Directive in Germany. Int. Rev. Hydrobiol. 2005, 90, 223–237. [Google Scholar] [CrossRef]
- Melzer, A. Aquatic macrophytes as tools for lake management. Hydrobiologia 1999, 395–396, 181–190. [Google Scholar] [CrossRef]
- Dobrokhotova, K.V. Chara algae in hydromacrophyte cenoses. Proc. All-Union Hydrobiol. Ova 1953, 5, 258–263. [Google Scholar]
- Kostin, V.A. Materials for the study of the ecology of charophytes of water bodies of the Ili-Balkhash Basin. Bot. Mater. Herb. Inst. Bot. Acad. Sci. SSR 1957, 15, 128–133. [Google Scholar]
- Kostin, V.A. Rare and endangered species of higher aquatic plants in water bodies of the river or Lake Balkhash. Bot. Mater. Herb. Inst. Bot. Acad. Sci. SSR 1983, 13, 111–116. [Google Scholar]
- Kostin, V.A.; Shoyakubov, R.S. Chara algae of Lake Balkhash and the influence of some environmental factors on their consumption. Algae Mushrooms Cent. Asia 1974, 1, 12–16. [Google Scholar]
- Krupa, E.G.; Barinova, S.S.; Tsoy, V.N.; Sadyrbaeva, N.N. Formation of phytoplankton of Lake Balkhash (Kazakhstan) under the influence of major regional-climatic factors. Adv. Biol. Earth Sci. 2017, 2, 204–213. [Google Scholar]
- Barinova, S.S.; Romanov, R.E. Towards an inventory of algal diversity of the Zerenda Lake, Northern Kazakhstan. In Biological Diversity of Asian Steppe, Proceedings of the III International Scientific Conference, Kostanay, Kazakhstan, 24–27 April 2017; Abil, E.A., Bragina, T.M., Eds.; KSPI: Kostanay, Kazakhstan, 2017; pp. 139–144. [Google Scholar]
- Romanov, R.E.; Kipriyanova, L.M.; Charitoncev, B.S. New speciesrecords of charophytes (charales, streptophyta) in West-Siberian plain (Russia). Bull. Mosc. Soc. Nat. Biol. Ser. 2017, 122, 67–70. [Google Scholar]
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M.; Khitrova, E.A. Hydrochemical and Hydrobiological Characteristics of the Lakes of the Shchuchinsko-Borovsk Resort Zone (Northern Kazakhstan) and the Main Methodological Approaches to Assessing the Ecological State of Small Water Bodies; Etalon Print: Almaty, Kazakhstan, 2021; p. 304. [Google Scholar]
- Jumakhanova, G.; Jiyenbekov, A.; Nurashov, S.; Sametova, E.; Shalgimbayeva, S. Variety of Chara algae in the Talgar River and its pond. Rep. Natl. Acad. Sci. Repub. Kazakhstan 2021, 1, 67–73. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. How a New Locality of Algal Community in the Negev Desert, Israel was formed. Expert Opin. Environ. Biol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Sametova, E.S.; Nurashov, S.B.; Shalgimbaeva, S.M.; Jiyenbekov, A.K.; Jumakhanova, G.B. Species composition of Chara Algae in The Talgar River and Ponds near Tuganbay village. In Proceedings of the Materials of the International Scientific and Practical Conference “The Modern Problems of Biology and Biotechnology”, Almaty, Kazakhstan, 27 May 2021; pp. 201–205. [Google Scholar]
- Dzhamangaraeva, A.K. Pliocene charophytes from Aktau Mountain, southeastern Kazakhstan. Geobios 1997, 30, 475–479. [Google Scholar] [CrossRef]
- Zhamangara, A.K. Charic Algae of the Middle Eocene of Kazakhstan. Bull. Karsu 2009, 1, 31–37. [Google Scholar]
- Sviridenko, B.F. Flora and Vegetation of Reservoirs of Northern Kazakhstan; Omsk State Pedagogical University: Omsk, Russia, 2000; pp. 96–102. [Google Scholar]
- Nurashov, S.B. The state of knowledge of the flora of charophytes of Kazakhstan. In Proceedings of the International Scientific Conference “Actual Problems of Algology, Mycology and Hydrobotany”, Tashkent, Uzbekistan, 11–12 September 2009; pp. 111–113. [Google Scholar]
- Nurashov, S.B.; Sametova, E.S. Analysis of the species composition of Chara algae in Kazakhstan. In IV International Conference, “Actual Problems of Modern Algology”; Algologia Supplement: Kyiv, Ukraine, 2012; pp. 218–219. [Google Scholar]
- Nurashov, S.B.; Sametova, E.S. Chara algae of the Ili-Balkhash basin. In Proceedings of the I(VII) International Conference on Aquatic Macrophytes, Borok, Russia, 9–13 October 2010; pp. 237–239. [Google Scholar]
- Nurashov, S.B.; Sametova, E.S. Chara algae of East Kazakhstan. In Botanical research in Asian Russia, Proceedings of the XI Congress Rus. Bot. Islands, 18–22 August 2003, Novosibirsk—Altay State University; AzBuka Publishing House: Barnaul, Russia, 2003; Volume 1, pp. 131–132. [Google Scholar]
- Jiyenbekov, A.K.; Nurashov, S.B.; Sametova, E.S.; Jumakhanova, G.B. Bioindicative assessment of the waters of the Chernaya River. Probl. Bot. South. Sib. Mong. 2021, 20, 160–168. [Google Scholar] [CrossRef]
- Jumakhanova, G.B.; Sametova, E.S.; Nurashov, S.B.; Jiyenbekov, A.K. Kegen and Rayimbek district Chara algae. In Materials of the International Scientific and Practical Conference “Independence of Kazakhstan: Aspects of Biodiversity Conservation”, 26 November 2021; Kazakh University: Almaty, Kazakhstan, 2021; pp. 205–207. [Google Scholar]
- Becker, R.; Doege, A.; Schubert, H.; van de Weyer, K. Bioindikation mit Characeen. In Armleuchteralgen; Springer Spektrum: Berlin/Heidelberg, Germany, 2016; pp. 97–137. [Google Scholar] [CrossRef]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 55558. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; 498p. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Kyiv, Israel, 2019; 367p. (In Russian) [Google Scholar]
- Khan, M. Charophytes in time and space: Zonal distribution pattern. Bulletin de la Société Botanique de France. Actual. Bot. 1991, 138, 33–45. [Google Scholar] [CrossRef]
- Puche, E.; Rojo, C.; Ramos-Jiliberto, R.; Rodrigo, M.A. Structure and vulnerability of the multi-interaction network in macrophyte-dominated lakes. Oikos 2020, 129, 35–48. [Google Scholar] [CrossRef]
- Climate-data.org. Climate Data for Cities Worldwide. Available online: https://en.climate-data.org/asia/kazakhstan/south-kazakhstan-province-2231/ (accessed on 20 March 2022).
- Climate-data.org. Available online: https://en.climate-data.org/asia/kazakhstan/jambyl-province-2238/ (accessed on 20 March 2022).
- Climate-data.org. Available online: https://en.climate-data.org/asia/kazakhstan/almaty-province-2251/ (accessed on 20 March 2022).
- Barinova, S. How to Align and Unify the Cell Counting of Organisms for Bioindication. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555–585. [Google Scholar] [CrossRef] [Green Version]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, 1. Teil, Chroococcales. In Süsswasserflora von Mitteleuropa, Band 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, E., Eds.; Gustav Fisher: Jena, Germany, 1999; 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota, 2. Teil, Oscillatoriales. In Süßwasserflora von Mitteleuropa, Band 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Spektrum Akademischer Verlag, Elsevier GmbH: München, Germany, 2005; 759p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 1, Naviculaceae. In Süsswasserflora von Mitteleuropa, Band 2/1; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1986; 876p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 2, Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, Band 2/2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1988; 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 3, Centrales, Fragilariaceae, Eunotiaceae. In Süsswasserflora von Mitteleuropa, Band 2/3; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1991; 598p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 4, Achnantaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In Süsswasserflora von Mitteleuropa, Band 2/4; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher: Jena, Germany, 1991; 468p. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. (Eds.) The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Alga; Cambridge University Press: Cambridge, UK, 2002; 702p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication; National University of Ireland: Galway, Ireland. Available online: http://www.algaebase.org (accessed on 24 June 2019).
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydroch. Hydrobiol. 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power Press: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Novakovsky, A.B. Abilities and base principles of program module “GRAPHS”. Sci. Rep. Komi Sci. Cent. Ural. Div. Russ. Acad. Sci. 2004, 27, 28. [Google Scholar]
- Newsletter on the State of the Environment of the Turkestan Region for the Month of February 2021; Ministry of Ecology, Geology and Natural Resources of the Republic of Kazakhstan RSE “Kazhydromet” Branch in the Turkestan region: Shymkent, Kazakhstan, 2021; 19p.
- Information Bulletin on the State of the Environment of the Republic of Kazakhstan; Ministry of Ecology, Geology and Natural Resources of the Republic of Kazakhstan Department of Environmental Monitoring RSE “Kazhydromet”: Nur-Sultan, Kazakhstan, 2019; Volume 1, 332p.
- Petrakov, I.A. Quality of Surface Waters on the Territory of the Republic of Kazakhstan for 2015; Department of Environmental Monitoring RSE “Kazhydromet”: Astana, Kazakhstan, 2015; 131p. [Google Scholar]
- Information Bulletin on the State of the Environment of the Republic of Kazakhstan; Ministry of Ecology Geology and Natural Resources of the Republic of Kazakhstan Department of Environmental Monitoring Rgp “Kazhydromet”: Nur-Sultan, Kazakhstan, 2020; Volume 1, 220p.
- Jumakhanova, G.B.; Jiyenbekov, A.K. History of study of chara algae in South and Southeast Kazakhstan. In Materials of the International Scientific Conference of Students and Young Scientists “Farabi World”, 6–9 April 2020; Kazakh University: Almaty, Kazakhstan, 2020; p. 37. [Google Scholar]
- Jiyenbekov, A.K.; Jumakhanova, G.B. Current Traffic Situation of Alakol Lake Water and Types of Nutrition of Algae Types. In Materials of the International Scientific Conference of Students and Young Scientists “Farabi World”, 6–9 April 2020; Kazakh University: Almaty, Kazakhstan, 2021; p. 36. [Google Scholar]
- Jumakhanova, G.B.; Jiyenbekov, A.K.; Nurashov, S.B.; Sametova, E.S. Varieties of Chara Algae in continental Water Reserves Near Almaty. In «Science and Education in the Modern World: Challenges of the XXI Century», VII International Scientific and Practical Conference, 20–22 October 2020; National movement ”Bobek”: Nur-Sultan, Kazakhstan, 2020; pp. 79–83. [Google Scholar]
- Jumakhanova, G.B.; Jiyenbekov, A.K.; Nurashov, S.B.; Sametova, E.S. Variety of chara algae in the Kaskelen River and Its Pond. In IV International Scientific Conference “Problems of Environmental Education in the 21st Century” Based on the Results, a Collection of Proceedings of the International Conference Will Be Published with the Assignment of ISBN and Included in the RSCI Database, 26 November 2020; Arkaim: Vladimir, Russia, 2020; pp. 50–54. [Google Scholar]
- Jiyenbekov, A.K.; Nurashov, S.B.; Sametova, E.S.; Jumakhanova, G.B.; Bigaliev, A.B. Peculiarities of Alakol Lake Algae types in the regional meeting. In Materials of the International Scientific and Practical Conference “Aspects and Innovations of Environmental Biotechnology and Bioenergy”, 12–13 February 2021; Kazakh University: Almaty, Kazakhstan, 2021; pp. 136–139. [Google Scholar]
- Jumakhanova, G.B.; Jiyenbekov, A.K. Various composition of algae in the talgar river and its reserves. In International Scientific Conference of Students and Young Scientists “Farabi World”, 6–7 April 2021; Kazakh University: Almaty, Kazakhstan, 2021; p. 33. [Google Scholar]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurasov, S.; Sametova, E. The first evidence about the algae of the protected Alakol Lake (Kazakhstan) and their floral analysis. Bull. Mosc. Soc. Nat. Dep. Biol. 2018, 123, 48–57. [Google Scholar]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurashov, S.; Sametova, E.; Fahima, T. Bioindication using diversity and ecology of algae of the Alakol Lake, Kazakhstan. Appl. Ecol. Environ. Res. 2018, 16, 7799–7831. [Google Scholar] [CrossRef]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurashov, S.; Sametova, E.; Fahima, T. Ecological diversity of algae in the Alakol Lake Natural Reserve, Kazakhstan. Bot. Pac. A J. Plant Sci. Conserv. 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Jiyenbekov, A.; Barinova, S.; Bigaliev, A.; Nurashov, S.; Sametova, E.; Fahima, T. Algal comparative floristic of the Alakol Lake Natural State Reserve and other lakes in Kazakhstan. MOJ Ecol. Environ. Sci. 2018, 3, 252–258. [Google Scholar] [CrossRef]
- Barinova, S.S.; Bragina, T.M.; Nevo, E. Algal species diversity of arid region lakes in Kazakhstan and Israel. Community Ecol. 2009, 10, 7–16. [Google Scholar] [CrossRef]
- Şahin, B.; Akar, B.; Barınova, S. Cohabitant charophyte algal flora and its ecology in high-mountain lakes of the Artabel Lakes Nature Park (Gümüşhane, Turkey). Bot. Serbica 2020, 44, 11–25. [Google Scholar] [CrossRef]
- Şahin, B.; Barinova, S. Assessment of Charophyta flora and ecological status in two high-mountain lakes (Rize, Turkey). Transylv. Rev. Syst. Ecol. Res. Wetl. Divers. 2022, 24, 35–54. [Google Scholar] [CrossRef]
- Barinova, S.; Sivaci, R. Experimental approach to a lake ecosystem assessment in the Great Lota, Turkey. Experiment 2013, 9, 566–586. [Google Scholar]
- Sivaci, R.E.; Barinova, S.; Solak, C.N.; Çobanoglu, K. Ecological assessment of Great Lota Lake (Turkey) on the base of diatom communities. Afr. J. Biotechnol. 2013, 12, 453–464. [Google Scholar] [CrossRef]
- Barinova, S.; Fatyukha, A.; Romanov, R. Macrophytes and Charophytes in ecological assessment of the protected lakes in Donetsk Region, Ukraine. Nat. Resour. Conserv. 2014, 2, 71–79. [Google Scholar] [CrossRef]
- Ali, A.; Badshah, L.; Barinova, S. Diversity and seasonal dynamics of Chlorophyta Reichenbach, 1928 and Charophyta Migula, 1980 algae in the Swat River basin, Pakistan. Bioresour. Environ. 2019, 2, 41–58. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S. The Charophytes of Israel: Historical and contemporary species richness, distribution, and ecology. Biodivers. Res. Conserv. 2012, 25, 67–74. [Google Scholar] [CrossRef]
- Yehuda, G.; Barinova, S.S.; Krugman, T.; Pavlicek, T.; Nov, Y.; Nevo, E. Microscale adaptive response of charophytes of the Negev Desert, Israel: Species divergences by AFLP. Nat. Resour. Conserv. 2013, 1, 55–64. [Google Scholar] [CrossRef]
- Barinova, S.; Romanov, R. Charophyte Community in the Lowermost Locality in the World near the Dead Sea, Israel. Int. J. Plant Soil Sci. 2015, 6, 229–243. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kohshima, S.; Ohtani, S. A Community of Snow Algae on a Himalayan Glacier: Change of Algal Biomass and Community Structure with Altitude. Arct. Alp. Res. 1997, 29, 126–137. [Google Scholar] [CrossRef]
- Uetake, J.; Naganuma, T.; Hebsgaard, M.B.; Kanda, H.; Kohshima, S. Communities of algae and cyanobacteria on glaciers in west Greenland. Polar Sci. 2010, 4, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Blaženčić, J.; Stevanović, B.; Blaženčić, Ž. Distribution and ecology of charophytes recorded. Cryptogam. Algol. 2006, 27, 311–322. [Google Scholar]
- Carson, J.L.; Brown, R.M., Jr. The Correlation of Soil Algae, Airborne Algae, and Fern Spores with Meteorological Conditions on the Island of Hawaii. Pac. Sci. 1976, 30, 197–205. [Google Scholar]
- Stewart, A.; Rioux, D.; Boyer, F.; Gielly, L.; Pompanon, F.; Saillard, A.; Thuiller, W.; Valay, J.-G.; Maréchal, E.; Coissac, E. Altitudinal Zonation of Green Algae Biodiversity in the French Alps. Front. Plant Sci. 2021, 12, 679428. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Patova, E.N.; Dubrovskiy, Y.A.; Novakovskiy, A.B.; Kulyugina, E.E. Distribution of algae and cyanobacteria of biological soil crusts along the elevation gradient in mountain plant communities at the Northern Urals (Russian European Northeast). J. Mt. Sci. 2022, 19, 637–646. [Google Scholar] [CrossRef]
- Vesić, A.; Blaženčić, J.; Šinžar-Sekulić, J. Ecological preferences of charophytes in Serbia in relation to habitat type and other aquatic macrophytes. Plant Biosyst. 2016, 150, 490–500. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S. Species of Nitella (Charophyceae, Charales) from Israel: Low species richness and rare occurrence. Bot. Serbica 2016, 40, 217–227. [Google Scholar] [CrossRef]
- Barinova, S. Plants, mosses, charophytes, protozoan, and bacteria water quality indicators for assessment of organic pollution and trophic status of continental water bodies. Transylv. Rev. Syst. Ecol. Res. Wetl. Divers. 2021, 23, 17–36. [Google Scholar] [CrossRef]
- Blindow, I. The composition and density of epiphyton on several species of submerged macrophytes—the neutral substrate hypothesis tested. Aquat. Bot. 1987, 29, 157–168. [Google Scholar] [CrossRef]










| Site | Basin | Name | North | East | Altitude (m) |
|---|---|---|---|---|---|
| 1 | I | * Canal Dostyk | 41°00′31.80″ | 68°12′40.43″ | 245 |
| 2 | I | Syrdarya River | 41°02′16.79″ | 68°30′49.94″ | 418 |
| 3 | I | Karatausky nature reserve Kizhi, source Karakuz | 43°51′07.48″ | 68°32′14.65″ | 971 |
| 4 | I | * Sharbulak River | 41°46′19″ | 69°24′10″ | 650 |
| 5 | I | * Merki River | 42°54′11.09″ | 73°09′51.17″ | 676 |
| 6 | II | * Theris River | 42°39′59″ | 70°48′05″ | 953 |
| 7 | II | Mynaral River | 45°24′49″ | 73°40′51″ | 343 |
| 8 | II | Chu River | 43°16′05″ | 74°12′13″ | 533 |
| 9 | II | * Karabalta River | 43°12′01″ | 74°0′36″ | 520 |
| 10 | II | Kakpatas River | 43°21′13″ | 74°24′48″ | 561 |
| 11 | II | * Dam Copa | 43°21′13″ | 74°28′45″ | 636 |
| 12 | II | Aksu River | 43°11′53″ | 74°3′48″ | 751 |
| 13 | III | * Ili River, Canal Arystan | 45°32′29″ | 74°52′42″ | 341 |
| 14 | III | * Ili River, Canal Zhidely | 45°33′00″ | 74°53′42″ | 341 |
| 15 | III | * Canal Bakanas | 44°52′50.37″ | 76°10′13.98″ | 389 |
| 16 | III | Lake Yubelejnoe | 43°20′31″ | 76°42′02″ | 696 |
| 17 | III | Lake Sorbulak | 43°38′01″ | 76°36′29″ | 618 |
| 18 | III | * Talgar River | 43°41′50″ | 77°15′25″ | 394 |
| 19 | III | Ostemir pond | 43°38′52″ | 77°15′48″ | 523 |
| 20 | III | * Kaskelen River pond | 43°46′27″ | 77°4′53″ | 488 |
| 21 | III | Ili-Kapchagaiplatinum | 43°55′7.49″ | 77°5′49.31″ | 475 |
| 22 | III | Kaskelen River | 43°47′03″ | 77°7′47″ | 623 |
| 23 | III | * Lake Kaiyndy | 42°59′05.58″ | 78°27′54.79″ | 1865 |
| 24 | III | * Karkara River | 42°50′57.64″ | 79°13′57.98″ | 2062 |
| 25 | III | * Mynzhylky River | 42°44′15.8″ | 79°16′53.7″ | 3000 |
| 26 | III | * Sartasu River | 42°37′14.49″ | 79°19′18.61″ | 3629 |
| 27 | III | * River Kegen | 43°00′27.64″ | 79°15′13.23″ | 1821 |
| 28 | III | Charyn River | 43°52′49.40″ | 79°27′13.56″ | 512 |
| 29 | III | * Ulken-Kokpak River | 42°36′06.68″ | 79°50′42.41″ | 1836 |
| 30 | III | * Tekes River | 42°50′37.1″ | 80°03′07.5″ | 1766 |
| 31 | III | * Narynkol River | 42°43′24.86″ | 80°08′06.54″ | 1831 |
| 32 | III | * Tentek River | 45°16′31.99″ | 80°73′75.03″ | 2338 |
| 33 | III | Alakol Lake | 46°40′77.51″ | 81°45′71.19″ | 347 |
| Score | Visual Estimate | Cell Numbers of Plankton per L | Cell Numbers of Periphyton per Slide (20 × 20 mm) | Cell Number of Each Species, % |
|---|---|---|---|---|
| 1 | Occasional | 1–103 cell L−1 | 1–5 cells per slide | <1 |
| 2 | Rare | 103–104 cell L−1 | 10–15 cells per slide | 2–10 |
| 3 | Common | 104–105 cell L−1 | 25–30 cells per slide | 10–40 |
| 4 | Frequent | 105–107 cell L−1 | 1 cell over a slide transect | 40–60 |
| 5 | Very frequent | 106–107 cell L−1 | Several cells over a slide transect | 60–80 |
| 6 | Abundant | More than 107 cell L−1 | One or more cells in each field of view | 80–100 |
| Basin | Site | Altitude, m a.s.l. | Temperature, °C | pH | O2, mg L−1 | BOD, mg O2 L−1 | Pt/Co Color Degree | Index S | No. of Species |
|---|---|---|---|---|---|---|---|---|---|
| Basin I | 4 | 650 | 27 | 7.00 | - | - | - | 2.07 | 35 |
| Basin II | 6 | 941 | 12 | 7.00 | - | - | - | 1.91 | 21 |
| 8 | 739 | 36 | 7.24 | 10.50 | 3.85 | 10.0 | 1.85 | 27 | |
| 10 | 561 | 30 | 7.00 | - | - | - | 1.87 | 46 | |
| 12 | 751 | 30 | 7.39 | 11.85 | 6.76 | 12.5 | - | 37 | |
| Basin III | 13 | 341 | 22 | 7.33 | 11.85 | 0.87 | 5.5 | 1.72 | 20 |
| 14 | 341 | 24 | 7.33 | 11.85 | 0.87 | 5.5 | 2.11 | 32 | |
| 15 | 396 | 24 | 7.00 | - | - | - | 1.61 | 40 | |
| 16 | 808 | 10 | 6.00 | - | - | - | 1.73 | 25 | |
| 17 | 632 | 35 | 7.75 | - | - | - | 2.00 | 12 | |
| 20 | 488 | 8 | 6.50 | - | - | - | 1.59 | 37 | |
| 21 | 475 | 21 | 7.44 | 12.10 | 1.09 | 5.5 | 1.96 | 31 | |
| 24 | 1900 | 4.5 | 7.43 | 12.15 | 1.43 | 6.0 | 1.73 | 16 | |
| 25 | 3000 | 14 | 7.00 | - | - | - | - | 13 | |
| 29 | 1836 | 4.5 | 7.00 | - | 0.65 | 0.0 | 1.26 | 45 | |
| 30 | 1766 | 3 | 7.76 | 11.35 | 0.85 | 6.0 | 1.72 | 36 | |
| 33 | 347 | 22 | 7.50 | - | - | - | 1.74 | 10 |
| Station | 13 | 14 | 33 | 15 | 21 | 20 | 10 | 17 | 4 | 8 | 12 | 16 | 6 | 30 | 29 | 24 | 25 |
| Altitude | 341 | 341 | 347 | 396 | 475 | 488 | 561 | 632 | 650 | 739 | 751 | 808 | 941 | 1766 | 1836 | 1900 | 3000 |
| Bacillariophyta | 8 | 12 | 6 | 21 | 14 | 21 | 21 | 5 | 19 | 15 | 30 | 16 | 15 | 19 | 35 | 13 | 8 |
| Charophyta | 3 | 5 | 4 | 6 | 3 | 8 | 7 | 3 | 3 | 4 | 4 | 4 | 2 | 6 | 7 | 3 | 3 |
| Chlorophyta | 5 | 6 | 0 | 3 | 9 | 1 | 14 | 2 | 5 | 2 | 0 | 3 | 2 | 2 | 2 | 0 | 0 |
| Cyanobacteria | 4 | 9 | 0 | 8 | 4 | 4 | 3 | 2 | 8 | 5 | 3 | 2 | 2 | 7 | 0 | 0 | 1 |
| Miozoa | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ochrophyta (Chrysophyceae) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ochrophyta (Xanthophyceae) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euglenozoa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 |
| No. Species | 20 | 32 | 10 | 40 | 31 | 37 | 46 | 12 | 35 | 27 | 37 | 25 | 21 | 36 | 45 | 16 | 13 |
| Index S | 2.11 | 1.72 | 1.74 | 1.61 | 1.96 | 1.59 | 1.87 | 2.00 | 2.07 | 1.85 | - | 1.73 | 1.91 | 1.72 | 1.26 | 1.73 | - |
| Habitat | |||||||||||||||||
| B | 5 | 14 | 6 | 16 | 9 | 19 | 15 | 6 | 14 | 14 | 21 | 12 | 7 | 16 | 24 | 10 | 6 |
| P-B | 12 | 13 | 3 | 18 | 16 | 11 | 20 | 4 | 19 | 11 | 11 | 12 | 12 | 13 | 18 | 6 | 6 |
| P | 2 | 2 | 0 | 2 | 4 | 1 | 6 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 |
| Temperature | |||||||||||||||||
| cool | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 4 | 1 | 0 |
| temp | 3 | 7 | 3 | 13 | 12 | 11 | 14 | 2 | 13 | 10 | 8 | 11 | 8 | 15 | 16 | 8 | 2 |
| eterm | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 2 | 3 | 0 | 2 |
| warm | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 0 |
| Oxygen | |||||||||||||||||
| aer | 0 | 2 | 0 | 3 | 2 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| str | 0 | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 3 | 0 | 0 |
| st-str | 11 | 14 | 7 | 19 | 17 | 16 | 24 | 5 | 21 | 13 | 12 | 15 | 12 | 20 | 26 | 10 | 6 |
| st | 1 | 3 | 1 | 4 | 2 | 5 | 6 | 1 | 1 | 3 | 2 | 3 | 4 | 1 | 3 | 1 | 2 |
| Watanabe | |||||||||||||||||
| sx | 0 | 0 | 1 | 3 | 1 | 5 | 3 | 0 | 3 | 2 | 5 | 3 | 4 | 5 | 9 | 3 | 1 |
| es | 5 | 8 | 4 | 11 | 10 | 9 | 11 | 1 | 11 | 7 | 8 | 9 | 7 | 9 | 16 | 7 | 3 |
| sp | 1 | 2 | 0 | 3 | 2 | 0 | 4 | 1 | 3 | 2 | 0 | 1 | 2 | 1 | 3 | 1 | 0 |
| Salinity | |||||||||||||||||
| hb | 1 | 1 | 0 | 1 | 0 | 3 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 2 | 4 | 1 | 0 |
| i | 6 | 9 | 4 | 21 | 17 | 16 | 22 | 3 | 18 | 17 | 17 | 16 | 11 | 18 | 24 | 9 | 7 |
| hl | 3 | 5 | 1 | 5 | 4 | 4 | 5 | 2 | 8 | 1 | 3 | 2 | 2 | 2 | 4 | 1 | 0 |
| mh | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 0 | 1 |
| hlbnt | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| eh | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| pH | |||||||||||||||||
| acb | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| acf | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 0 |
| ind | 5 | 5 | 1 | 10 | 7 | 6 | 14 | 0 | 11 | 13 | 8 | 5 | 7 | 10 | 18 | 5 | 4 |
| alf | 4 | 9 | 4 | 16 | 11 | 15 | 15 | 5 | 13 | 6 | 14 | 12 | 10 | 12 | 18 | 8 | 4 |
| alb | 2 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 3 | 1 | 0 | 0 |
| Autotrophy-Heterotrophy | |||||||||||||||||
| ats | 1 | 1 | 1 | 4 | 3 | 9 | 6 | 2 | 4 | 5 | 5 | 2 | 2 | 4 | 13 | 1 | 3 |
| ate | 4 | 6 | 3 | 12 | 8 | 8 | 8 | 1 | 8 | 5 | 7 | 11 | 8 | 11 | 16 | 8 | 1 |
| hne | 1 | 4 | 1 | 4 | 1 | 2 | 5 | 2 | 5 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | 2 |
| hce | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 1 | 2 | 1 | 0 | 1 | 0 |
| Trophy | |||||||||||||||||
| ot | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 2 | 2 | 3 | 0 | 1 | 1 | 5 | 1 | 0 |
| om | 0 | 1 | 2 | 7 | 1 | 4 | 5 | 1 | 4 | 4 | 2 | 1 | 2 | 5 | 8 | 0 | 1 |
| m | 1 | 6 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 3 | 0 | 4 | 3 | 4 | 1 |
| me | 3 | 0 | 3 | 7 | 5 | 10 | 5 | 0 | 8 | 7 | 0 | 4 | 2 | 8 | 6 | 0 | 1 |
| e | 7 | 13 | 3 | 11 | 12 | 6 | 15 | 5 | 15 | 5 | 7 | 6 | 8 | 4 | 9 | 4 | 3 |
| o-e | 1 | 2 | 2 | 3 | 1 | 3 | 2 | 2 | 0 | 2 | 5 | 2 | 0 | 2 | 1 | 2 | 1 |
| he | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Class of Water Quality | |||||||||||||||||
| Class 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 5 | 0 | 0 |
| Class 2 | 2 | 6 | 5 | 15 | 9 | 16 | 17 | 4 | 11 | 11 | 12 | 13 | 6 | 15 | 21 | 7 | 5 |
| Class 3 | 7 | 14 | 2 | 12 | 13 | 5 | 14 | 1 | 10 | 7 | 5 | 7 | 6 | 10 | 10 | 5 | 5 |
| Class 4 | 1 | 4 | 2 | 3 | 4 | 4 | 3 | 1 | 6 | 3 | 2 | 1 | 4 | 2 | 1 | 1 | 0 |
| Class 5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sametova, E.; Jumakhanova, G.; Nurashov, S.; Barinova, S.; Jiyenbekov, A.; Smith, T. Microalgae Indicators of Charophyte Habitats of South and Southeast Kazakhstan. Diversity 2022, 14, 530. https://doi.org/10.3390/d14070530
Sametova E, Jumakhanova G, Nurashov S, Barinova S, Jiyenbekov A, Smith T. Microalgae Indicators of Charophyte Habitats of South and Southeast Kazakhstan. Diversity. 2022; 14(7):530. https://doi.org/10.3390/d14070530
Chicago/Turabian StyleSametova, Elmira, Gaukhar Jumakhanova, Satbay Nurashov, Sophia Barinova, Aibek Jiyenbekov, and Thomas Smith. 2022. "Microalgae Indicators of Charophyte Habitats of South and Southeast Kazakhstan" Diversity 14, no. 7: 530. https://doi.org/10.3390/d14070530
APA StyleSametova, E., Jumakhanova, G., Nurashov, S., Barinova, S., Jiyenbekov, A., & Smith, T. (2022). Microalgae Indicators of Charophyte Habitats of South and Southeast Kazakhstan. Diversity, 14(7), 530. https://doi.org/10.3390/d14070530