3.3. Plagiothecium roeseanum
Schimper [
18] described
Plagiothecium roeseanum Hampe
ex Schimp. based on the unpublished
Hypnum roeseanum Hampe. In the diagnosis that Schimper gave, these plants are characterized by erect, julaceous, slightly flattened stems; ovate-lanceolate, gently imbricate leaves and almost erect capsules (
Figure 6).
Some 25 years after
P. roeseanum was described, Schimper [
20] wrote this taxon as
P. röseanum. However, this change (“oe” to “ö”) is only a Germanization for the double-character “oe” in the word
roeseanum. This way of writing is quite rare in the literature and appears only in a few studies, e.g., in
Index Bryologicus [
55].
For the next decades,
Plagiothecium roeseanum was used in the literature, e.g., [
10,
15,
33,
37,
40,
56], thus replacing the earlier described
H. cavifolium [
4]. Nevertheless, the understanding of this taxon by individual researchers varied greatly, e.g., Limpricht [
40], Mönkemeyer [
37] and Barkman [
15] interpreted this taxon very narrowly, describing, e.g., that its turf is dense and glossy; its stems are erect or creeping, julaceous, densely foliate; its leaves are imbricate, concave, symmetrical, and entire (non-serrate); its costae are double, very short, reaching 1/5–¼ leaf length with long and narrow cells.
On the other hand, Warnstorf [
33] and Jedlička [
10] understood this taxon more broadly, sometimes very broadly, but still separately from other species, including
Plagiothecium sylvaticum. The mentioned researchers reported a very wide range of variability of many taxonomic features of this species, indicating, e.g., that the turf is julaceous or slightly flattened, rarely completely flat; its leaves are mostly symmetrical and very concave or more asymmetrical and less concave and its leaf margins are serrate or not. In Jedlička [
10], this concept was particularly reflected in the 21 varieties, forms and subforms of this taxon described by him.
Plagiothecium roeseanum was understood differently by Walther and Molendo [
57] and Héribaud [
34], who treated it as a variety of
P. sylvaticum—P. silvaticum var.
roeseanum (Hampe
ex Schimp.) Hérib. A similar approach was adopted by Lindberg [
21] and Jensen [
47], except that the first of them wrote the name incorrectly as
P. silvaticum γ roesei (Hampe) Lindb. Thus, the orthographic variant “
roesei” appeared in many contemporary studies, e.g., [
24,
25,
26,
27,
28], and the taxon itself was treated as a subspecies—
P. sylvaticum subsp.
roesei (Lindb.) Kindb. or a separate species—
P. roesei (Lindb.) Milde [
24] (
Figure 7).
In addition, Braithwaite [
35] in his
British moss-flora adopted Lindberg’s [
21] point of view regarding this taxon as a variety of
Plagiothecium sylvaticum, as
P. silvaticum var.
β roesii (Hampe) Braithwaite. The author also made a mistake in the name of basionym of this species, writing it as
Hypnum roesii (Hampe) Braithwaite [
35].
The end of the 19th and the beginning of the 20th century are the times when many new varieties and forms of
Plagiothecium roeseanum were described. One of them given by Gravet was
P. roeseanum fo.
laxa Gravet
in Warnstorf, and another by Ruthe [
32] was
P. roeseanum var.
propaguliferum Ruthe
in Warnstorf. On the other hand, Warnstorf [
33] also treated this taxon as a form but writing it as
P. roeseanum fo.
propagulifera (Ruthe) Warnst.
In his studies, Warnstorf [
33,
54] proposed further varieties of this taxon:
Plagiothecium roeseanum var.
flagellaceum Warnst.,
P. roeseanum var.
angustirete Warnst. and
P. roeseanum var.
heterophyllum Warnst. The first two were characterized as plants sometimes with thin, flagellate stems and symmetrical, concave leaves, while
P. roeseanum var.
heterophyllum was described as a plant with flattened, but not julaceous stems; asymmetric, less concave, broadly-ovoid leaves and as a plant similar to
P. sylvaticum and occupying a similar habitat to that species.
Some 21 years later, Mönkemeyer [
37] proposed a new combination of the variety (
Plagiothecium roeseanum var.
flagellaceum) described by Warnstorf [
33], changing its status to
P. roeseanum fo.
flagellacea (Warnst.) Mönk. In his description, Mönkemeyer [
37] emphasized, as Warnstorf [
33] wrote earlier, that these plants are characterized by long, flagellate stems. The combination proposed by Mönkemeyer [
37] was adapted by Jedliček [
10], but he wrote it as did Warnstorf [
33]—
P. roeseanum fo.
flagellaceum. Moreover, he proposed a subform for this taxon—
P. roeseanum fo.
flagellaceum subfo.
propaguliferum Jedl., which was characterized only by the presence of gemmae [
10].
In the middle of the 20th century Jedlička [
10] proposed a change in the status of the varieties described above, e.g.,
P. roeseanum var.
angustirete as
P. roeseanum fo.
angustirete (Warnst.) Jedl.;
P. roeseanum var.
heterophyllum as
P. roeseanum fo.
heterophyllum (Warnst.) Jedl. and
P. roeseanum var.
densum as
P. roeseanum fo.
densum (Warnst.) Jedl., where he pointed out that the latter is characterized by, e.g., julaceous, erect stems and very concave, serrate leaves [
10].
At almost the same time, Warnstorf [
32,
33] and Mönkemeyer [
36] proposed a new taxon—
Plagiothecium roeseanum var.
julaceum Mönk.—which was characterized as a plant with thick, julaceous stems. However, nine years later Cardot [
41] also described a taxon with the same name—
P. roeseanum var.
julaceum Cardot (
Figure 8). Moreover, this author proposed this variety of the described species—
P. roeseanum var.
japonicum. A new variety is also given by Podpěra [
38]—
P. roeseanum var.
basalticum Podp., which more than half a century later he changed its status to the rank of form—
P. roeseanum fo.
basalticum (Podp.) Podp., describing that these plants, e.g., have a loose mesh of cells [
39]. The following decades brought a new form described by Mönkemeyer [
37],
P. roeseanum fo.
umbrosa Mönk., which is characterized as a plant with julaceous foliage but almost flattened at the top of stems.
The mid-20th century was a time when many new combinations of
Plagiothecium roeseanum were proposed, with the majority of changes related to transferring taxa at the varietal status to that of form. One of the authors who described many of the types of these new combinations has already been mentioned—Jedlička [
10]. His concepts concerned the taxa mentioned above, and also
P. silvaticum var.
cryptarum (Renauld and Hérib.) P.Syd. as
P. roeseanum fo.
cryptarum (Renauld and Hérib.) Jedl. and
P. sylvaticum var.
filiforme Broeck as
P. roeseanum fo.
filiforme (Broeck) Jedl. On the other hand, in the case of
P. roeseanum, Jedlička [
10] also proposed a change to
P. röseanum var.
gracile Breidler as
P. roeseanum fo.
gracile (Breidler) Jedl. He described this taxon as plants with loose turf; delicate, long stems and small non-imbricate leaves [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Moreover, Warnstorf [
33] and Mönkemeyer [
37] indicated that it is a montane species; additionally, Mönkemeyer [
37] and Jedlička [
10] reported that it is characterized by round or almost round decurrency cells.
The next combinations proposed by Jedliček [
11] were related to the change in the status of the varieties described by Kern [
42] and Meylan
in Amann [
58]. The first concerns the change of status of
Plagiothecium roeseanum var.
alpinum Kern to
P. roeseanum fo.
alpinum (Kern) Jedl., which he then proposed as a separate species—
P. alpinum (Kern) Jedl. Both authors described the plants as alpine, with julaceous stems, very wide and very concave leaves and short costae.
The second combination referred to
Plagiothecium roeseanum var.
subjulaceum Meylan and its change to the form level—
P. roeseanum fo.
subjulaceum (Meylan) Jedl. and its subsequent recognition as an independent species,
P. subjulaceum (Meylan) Jedl. [
11]. Jedlička [
10,
11] described these plants as dense, yellowish or dark green, slightly shiny turf; with short, julaceous, densely foliate stems and broadly ovoid-lanceolate, very concave and folded leaves. However, 11 years later [
48] this species was reduced in rank, and once again it reappeared as
P. roeseanum fo.
subjulaceum.
Apart from proposing new combinations, Jedlička [
10] described a number of new taxa for
P. roeseanum, including the new forms:
P. roeseanum fo.
rigidum Jedl., which he described as plants with dense, yellowish-green, shiny turf, julaceous, densely foliate stems and very concave, serrate leaves;
P. roeseanum fo.
tenue Jedl. (
Figure 9) was characterized as creeping, dirty green turf plants, with filamentous, non-imbricate stems and flat leaves [
10]. For this taxon, Jedlička [
10] also proposed a subform—
P. roeseanum fo.
tenue subfo.
propaguliferum Jedl., which is distinguished only by the presence of gemmae. Some 13 years later, Pilous in Jedlička [
48] proposed another subform for this taxon—
P. roeseanum fo.
tenue subfo.
gemmicladum Pilous with which he synonymized
P. roeseanum fo.
tenue subfo.
propaguliferum previously proposed by Jedlička [
10].
Plagiothecium roeseanum fo.
subdentatum Jedl. (
Figure 10) is yet another form proposed by Jedlička [
10]; he characterized this taxon as plants with dense, yellow-green, shiny turf; short, julaceous, densely foliate stems and very concave leaves with a serrate apex. Another taxon at the rank of form proposed by Jedliček [
10] is
P. roeseanum fo.
strenuum Jedl., in the description of which the author stated that the plants are characterized by dark green, shiny, julaceous and densely foliate turf with large, ovate-lanceolate, concave or nearly flat leaves. Jedlička [
10] added that it resembles
P. platyphyllum Mönk. and proposed a subform—
P. roeseanum fo.
strenuum subfo.
propaguliferum Jedl., stating that it is characterized only by the presence of gemmae [
10]. Two years later, Jedlička [
11] changed the status of
P. roeseanum fo.
strenuum as
P. strenuum (Jedl.) Jedl. Nevertheless, 11 years later, Pilous
in Jedlička [
48] still distinguished this taxon proposed by Jedlička [
10] at the form level, for which Pilous
in Jedlička [
48] proposed a subform—
P. roeseanum fo.
strenuum subfo.
moravicum Pilous.
The taxa related to
Plagiothecium sylvaticum and
P. roeseanum have also been described as related to
P. denticulatum. Grout [
44] treated the latter as a subspecies—
P. denticulatum subsp.
roeseanum (Bruch and Schimp.) Grout, which is characterized as plants with julaceous stems and imbricate leaves. Both the abovementioned authors understood
P. denticulatum quite broadly, but Grout [
44] very broadly, because within this species he also included a number of other taxa as subspecies, including
P. sylvaticum,
P. ruthei,
P. laetum and the abovementioned
P. roeseanum.
3.6. Grouping of Taxa and the Taxonomic Implications
As the above list shows, the analyzed taxa sometimes differ diametrically from each other in terms of many, even taxonomically significant, features. Thus, they do not seem to belong to the Plagiothecium cavifolium complex. Others, in terms of the features included in the diagnoses, seem to be more or less consistent with the diagnosis of H. cavifolium.
In terms of the analyzed features, the original materials and the analyzed names quite easily form distinguishable groups (
Figure 11). Nevertheless, in the grouping below, some of them have been omitted due to the lack (for example in diagnoses) of all the necessary information for the analysis data or lack of a possibility to obtain original materials with these names.
The first group includes taxa characterized by julaceous stems; imbricate symmetrical, concave, non-serrate and more or less folded leaves; the cells from the middle part of the leaf larger than 101 µm in length and inclined capsules. This group contains
P. attenuatirameum (PC0132687),
P. roeseanum (
Hypnum roeseanum) (
Figure 11 and
Figure 12) (JE04004196, JE04004197, JE04004198, JE04004199),
P. roeseanum var.
angustirete (JE4004200) and
P. roeseanum var.
japonicum (PC0132574) (
Figure 11). On the basis of diagnoses and figures assuming this set of features, in the above group we can also include
P. orthocladium. The abovementioned features fit perfectly with the diagnosis of
H. cavifolium and with the description of
P. cavifolium sensu stricto, thus we propose to consider these taxa as synonyms of
P. cavifolium.
Plagiothecium cavifolium (Brid.) Z.Iwats., J. Hattori Bot. Lab. 33: 360 (1970); Hypnum (Stereodon) cavifolium Brid., Bryologia Universa 2: 556 (1827) (“cavifolius”; Stereodon cavifolius (Brid.) Brid., Bryologia Universa 2: 824 (1827). Type: in terra habitat in insula Terre Neuve, La Pylaie (B-Brid 915).
Plagiothecium roeseanum Hampe ex Schimp., Bryologia Europea 5: 193, 504 (Table X) (1851); Hypnum roeseanum Hampe in Bruch, Schimper and W.Gümbel, Bryologia Europea 5: 193, 504 (1851); P. sylvaticum var. roeseanum (Hampe ex Schimp.) A.W.H.Walther and Moldendo, Laubm. Oberfrank. 177 (1868); P. denticulatum var. roeseanum (Hampe ex Schimp.) Hérib., Mém. Acad. Sci. Clermont-Ferrand, sér. 2, 14: 228 (1899); P. denticulatum subsp. roeseanum (Hampe ex Schimp.) Grout, Moss Fl. N. Amer. 3: 158 (1932). Type: Ad terram arenosam sub Fagis in monte Inselberg Thuringiae cl. A. Roese legit atque nobiscum benevole communicavit (JE04004196!, JE04004197!, JE04004198!, JE04004199!).
Plagiothecium orthocladium Schimp., Bryologia Europea 5: 193, 504 (Table X) (1851); P. sylvaticum var. orthocladium (Schimp.) Schimp., Corollarium Bryologiae Europaeae 115 (1856); Hypnum sylvaticum var. orthocladium (Schimp.) Husn., Fl. Mousses Nord. Ouest (ed. 2) 149 (1882); P. roeseanum var. orthocladium (Schimp.) Limpr., Laubm. Deutschl. 3: 262 (1897); P. denticulatum var. orthocladium (Schimp.) Hérib., Mém. Acad. Sci. Clermont-Ferrand, sér. 2, 14: 229 (1899); P. sylvaticum fo. orthocladium (Schimp.) Barkman, Phytosociol. Ecol. Cryptog. Epiphytes 619 (1958); P. cavifolium var. orthocladium (Schimp.) Z.Iwats., J. Hattori Bot. Lab. 33: 371 (1970). Type: In m. Donnersberg Vogesi inferioris, Th. Gumbel legit auno 1842 (n.v.).
Plagiothecium attenuatirameum Kindb., Catalogue of Canadian Plants, Part VI, Musci 277 (1892); P. laetum subsp. attenuatirameum (Kindb.) Kindb., Canad. Rec. Sci. 6(2): 72 (1894). Type: Canada, Québec, Chelsea in Gilmour’s Park, on rock, J. Macoun 417, 6 September 1889, herb. I. Thériot (PC0132687!).
Plagiothecium roeseanum var. angustirete Warnst., Verh. Bot. Vereins Prov. Brandenburg 42: 214 (1900); P. roeseanum fo. angistirete (Warnst.) Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 39 (1948). Type: Germany, Brandenburg, Chorin (Mark), Hohlweg am Bach, am Waldhohlwege im „Forstarten” mit Eurhynchium schleicheri, L. Loeske, 10 Sep. 1899, herb. H. Dohl (JE4004200!).
Plagiothecium roeseanum var. japonicum Cardot, Bull. Soc. Bot. Genève, sér. 2, 4: 385 (1912). Type: Japan, Aomori Pref., Faurie 408 (“P. silvaticum var. orthocladum Sch.”), herb. J. Cardot (PC0132574!); idem, Faurie 418; Kanita, Faurie 1812; Hirosaki, Faurie 1878; Osorezan, Fauire 2104; château d’Akita, Faurie 2904; Nayoro, Faurie 3078 in parte; Sambongi, Faurie 3190; Otaru, Faurie 3753; Tobetsu, Faurie 3761 (KYO).
Taking into account the analyzed features,
P. propaguliferum (PC0132610) (
Figure 13) could be considered a new synonym for
P. cavifolium sensu lato; however, the difference from the diagnosis of
H. cavifolium confirms the legitimacy of excluding this name from the described complex. Additionally, with
P. apiculatum (MAK B115140) and
P. roeseanum var.
alpinum (PC0132603), they form another group of specimens characterized by symmetrical, concave, more or less folded leaves, with cells of the central part of the leaf longer than 101 µm. However, these specimens differ from the previous group in the serration of the leaf apex. Assuming this set of features, for this group we can also include
P. ikegamii,
P. roeseanum fo.
rigidum,
P. roeseanum fo.
subdentatum,
P. roeseanum fo.
alpinum and
P. alpinum. Thus, taking into account the analysis of original materials and diagnoses, we propose to resurrect
P. ikegamii as a separate species and consider the other taxa mentioned above as its synonyms.
Plagiothecium ikegamii Sakurai, Botanical Magazine (Tokyo), 62: 113, f. 3. (1949). Type: Japan, Etigo Prov., Mt. Renge, ad terram, ca. 2200 m, Y. Ikegami 11270, herb. K. Sakurai 16336, August 1949; Shinano Prov., Mt. Shirouma, 2500 m, N. Takaki in herb. K. Sakurai 16368, August 1949 (n.v.).
Plagiothecium roeseanum var. alpinum Kern, Jahresber. Schles. Ges. Vaterl. Cult. 91(2b): 64 (1914); P. roeseanum fo. alpinum (Kern) Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 37 (1948); P. alpinum (Kern) Jedl., Spisy Přír. Fak. Masarykovy Univ. 318: 5 (1950). Type: Italy, Felsritzen des Cruschettapasses an der Schweizer Grenze, 2300 m, F. Kern, 30 July 1913 (PC0132603!).
Plagiothecium roeseanum fo. rigidum Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 37 (1948). Type (authentic specimens cited in Jedlička 1961): Moravia, Jeseníky, Švýcárna, 1300 m, ster., J. Podpěra, H. M. B.; Brno, Bílovice, cfr., K. Doležal, H. U. B., s. P. denticulatum; Adamov, in conc. riv. Kateřinský, ster., J. Jedlička, H. J.; Slovakia, Vysoké Tatry, Štrbské Solisko, in Calamagrostideto villosae, solo granitico, 1385 m, ster., Krajina, H. U. P., sub P. denticulatum (n.v.).
Plagiothecium roeseanum fo. subdentatum Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 38 (1948); P. subdentatum (Jedl.) Jedl., Spisy Přír. Fak. Masarykovy Univ. 318: 5 (1950). Type (authentic specimens cited in Jedlička 1961): Moravia, Jeseníky, ster. cum Desmatodon, Frank H. P., Inter. p. Dalečín et Jimramov, 500 m, ster., J. Podpěra, H. P.; Carp. occid., Rožnov, s. m. Radhošt, versus Kluzov, ster., J. Podpěra, H. P.; Turcia, Salonichi, Kartaš-dagh, 1200 m, ster., J. Podpěra, H. P. (n.v.).
Plagiothecium propaguliferum Broth., in sched. Basis: Japan, Sendai, Y. Iishiba, July 1907, herb. J. Cardot, I. Thériot (PC0132610!).
Plagiothecium apiculatum Sakurai, in sched. Basis: Japan, Niigata Pref., Toyanao, Y. Ikegami 4256, 2 Apr. 1942 (MAK B115140!).
The quantitative and qualitative characteristics of the original materials also indicate that
P. sylvaticum var.
latifolium (HBG21134) and
P. succulentum var.
longifolium (JE 4004211, JE 4004212) can be synonymous with
P. cavifolium sensu lato. However, compared to the diagnosis of
H. cavifolium, the morphological diversity of these taxa confirms the legitimacy of excluding them from the described complex. The abovementioned specimens, together with
P. fujiyamae (MAK 57198),
P. nakajimae (MAK B57158),
P. roeseanum fo.
umbrosa (
Figure 14) (HBG021131),
P. succulentum var.
longifolium (JE 4004211, JE 4004212) and
P. sylvaticum var.
cavifolium (PC0132571) form one group of specimens characterized by julaceous stems; imbricate, symmetrical, strongly concave, non-serrate and more or less folded leaves and with inclined capsules. However, they are distinguished from the first group by shorter cells, the length of which does not exceed 100 µm. Assuming this set of features to the above group, we can also include
P. roeseanum var.
subjulaceum,
P. roeseanum fo.
subjulaceum, and
P. subjulaceum. Thus, taking into account the analysis of original materials and diagnoses, we propose to resurrect
P. subjulaceum as a separate species and consider the other taxa mentioned above as its synonyms.
Plagiothecium subjulaceum (Meyl.) Jedl., Spisy Přír. Fak. Univ. Masarykovy Univ. 318: 5 (1950); P. roeseanum var. subjulaceum Meyl. in J.J. Amann, Fl. Mouss. Suisse 2: 328 (1918); P. roeseanum fo. subjulaceum (Meyl.) Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 38 (1948). Type: No type was specified.
Plagiothecium roeseanum fo. umbrosa Mönk., Laubm. Eur. 863 (1927). Type: Germany, Thüringen, Finsteres Loch, Rich Schmidt Lips., 20 June 1916 (HBG021131!).
Plagiothecium sylvaticum var. cavifolium Jur. in Rabenhorst, Bryotheca Europaea 16: 765 (1864). Type: Bryotheca europaea 765, Auf nacktem Boden in Buchenwäldern auf Nagelfluhe am Mönchsberge bei Salzburg, Sauter (als. Plag. Lucens Sauter n. sp.), distrib. L. Rabenhorst (FH220150, MO406590, PC00132571!).
Plagiothecium silvaticum var. latifolium Röll, Deutsche Bot. Monatsschr. 9: 131 (1891), non Cardot, Bull. Soc. Bot. Genève, sér. 2, 4: 385 (1912), hom. illeg.; P. sylvaticum var. latifolium Röll, Hedwigia 56: 229 (1915), hom. illeg. Type: Germany, Thuringia, im Werrthal bei Plankenburg an der hohen Schlaufe bei Ilmenau, J. Röll (HBG21134!).
Plagiothecium succulentum var. longifolium Mönk., Laubm. Eur. 863, f. 206b (1927); P. sylvaticum fo. longifolium (Mönk.) C.E.O.Jensen, Skand. Bladmossfl. 495 (1939); P. succulentum fo. longifolium (Mönk.) Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 42 (1948). Lectotype (designated here): Germany, Thüringen Wald, am Simmetsberg im Ungeheuren Grund, Hess, Aug. 1872 (JE 4004211!), isolectotype: Germany, Thüringen, Annathal bei Eisenach, Hess, Aug. 1872 (JE 4004212!).
Plagiothecium fujiyamae Sakurai, in sched. Basis: Japan, Aokigahara, Fuji, Yamanashi Pref., T. Maede 1462, 9 Nov. 1950, herb. K. Sakurai (MAK 57198!).
Plagiothecium nakajimae Sakurai, in sched. Basis: Japan, Chichinu, Nagano, 6 Nov. 1951, herb. K. Sakurai 761 (MAK B57158!).
The next group is created by
Leskea flaccida (B31076701) (
Figure 15), and it represents materials with julaceous stems; imbricate, symmetrical, concave, more or less folded leaves; with the cells of the central part of the leaf shorter than 150 µm. It differs from the rest of the groups by erect capsules. Taking this set of features as a determinant, the analysis of original materials also allows the inclusion in this group of
H. sullivantiae (PC0132606, PC0132607, PC0132608) and
P. sullivantiae, while the analysis of diagnoses of individual names also allows the inclusion of
P. roeseanum var
. orthocladon fo.
propaguliferum and
P. roeseanum var
. orthocladon fo.
moravicum. Taking the above into account, we propose a new combination for this taxon
Plagiothecium flaccidum (Brid.) G.J.Wolski and W.R.Buck comb. nov., and we propose to consider the abovementioned names as synonyms of this taxon.
Plagiothecium flaccidum (Brid.) G.J.Wolski and W.R.Buck, comb. nov. Leskea flaccida Brid., Bryologia Universa 2: 308 (1827). Type: In Republica Massachusets Americae Foedewatae circa Noveboracum in rupis habitat, caespitosa, caespitum basi e congerie caulium veterarnorum marcescentium constante, Torrey 67, 1820 (B31076701!).
Hypnum sullivantiae Schimp. ex Sull., Manual (ed. 2) 680 (1856); Plagiothecium sullivantiae (Schimp. ex Sull.) Schimp. ex A.Jaeger, Ber. Thätigk. St. Gallischen Naturwiss. Ges.t 1876–77: 450 (1878); P. sylvaticum var. sullivantiae (Schimp. ex Sull.) Renauld and Cardot, Rev. Bryol. 20: 22 (1893). Type: Ohionis et Novae Angliae, in rupium fissuris terra impletis, Musci Boreali-Americani 355 (PC0132606!, PC0132607!); idem Herb. M. Bizot 13157 (PC0132608!).
Plagiothecium roeseanum var. orthocladon fo. propaguliferum Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 39 (1948), hom. illeg., non (R.Ruthe) Jaap, Verh. Naturwiss. Vereins Hamburg, ser. 3, 7: 36 (1900); P. roeseanum var. orthocladon fo. moravicum Pilous in Jedlička, Spisy Přír. Fak. Univ. v Brně 422: 214 (1961), nom. nov. Type: Moravia, conv. flum. Oslava, ster., Latzel, H.L., observavi (n.v.).
The analysis of the diagnoses of the remaining taxa also shows that this respect of features differs significantly from the diagnosis of H. cavifolium. Sometimes the differences are importance and basic. Thus, some of the analyzed names were described as not julaceous and characterized by flat, more or less asymmetrical leaves. Taking these features as diagnostic, the analysis of protologues in this group allowed the inclusion of P. roeseanum fo. acuminatum, P. cavifolium fo. acuminatum, P. roeseanum fo. Tenue and P. roeseanum fo. tenue subfo. propaguliferum. Taking into account the above and the fact that the oldest name for this group is P. roeseanum fo. tenue, we propose a new combination for this taxon—Plagiothecium tenue (Jedl.) G.J.Wolski and W.R.Buck comb. nov. and we propose to consider all the abovementioned taxa as synonyms of this species.
Plagiothecium tenue (Jedl.) G.J.Wolski and W.R.Buck comb. nov.; P. roeseanum fo. tenue Jedl., Spisy Přír. Fak Masarykovy Univ. 308: 38 (1948). Type (authentic specimens cited in Jedlička 1961): Silesia, Cuidowa, Steinberg, ster. Paul, H.M.B.; Bohemia, Beroun, Skryje, in decl. Vosník col. ster., Šmerda, H. Š. (sub P. denticulatum). Moravia, Jeseníky, Quarklöcher, pr. Brummlitz, ster. una cum Barbula rigida et Fissidens pusillus, Latzel, H. L.; Voskovice, in silva umbrosa pr. oppid, 300 m, ster., Doležal, H. P.; Brno, Kuřím, ad col. Baba, ster. Doležal, H. M. B. (sub P. denticulatum); Kůňku pr. Obora, str., Podpěra, H. P.; Mor. Krumlov, ad rup. perm., 300 m, ster. Podpěra, H. M. B.; Carp. occid., in m. Ondřejník, pr. Frýdlant, ster., Podpěra H. P.; in m. Lysá in conv. riv. Mazák, ster., Podpěra, H. P.; Rajnochovice, Pomsko, ster., Podpěra, H. P.; Rychtářov, in conv., V. Haná, ster., Podpěra, H. P.; Unčov, cataract. Řešovský, ster., Podpěra, H. P. Austria. Koralpe, Theisseneppergraben, solo granit., 800 m, ster., Latzel, H. L.; Pressinggraben, ster. Latzel, H. L. (s. P. Roeseanum gracile). Jugoslavia, Surdulica, in conv. Vrla reka, ster. Podpěra, H. P.; Vrane-Kazandžol, ster., Podpěra, H. P (n.v.).
Plagiothecium roeseanum fo. tenue subfo. propaguliferum Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 38 (1948), hom. illeg.; P. roeseanum subfo. gemmicladum Pilous, Spisy Přír. Fak. Univ. v Brně 422: 212 (1961), nom. nov. Type (authentic specimens cited in Jedlička 1961): Suecia, Skåne, Bokeberg, ster., Möller, H. M. B. Germania, Sachsen, Plauen, ad saxa umber. in conv. Elstertal, ster., Stolle, H. P. (planta pulcherima!!). Austria, Saualpe, Pöllinggraben, cfr., Latzel, H. L. Wien, ad arcem Greifenstein, 300 m, cfr., Baumgartner, Krypyog. exsicc. M. N. no. 1788a, H. M. P. Bohemia, Praha, Hasenburg, 250 m, ster., Bauer; Musc. eur. exsicc. no. 1311, H. P., H. M. B., H. M. P., H. U. B. (sub P. Roeseanum fo. gracilescens) Bauer in sched.; Řevnice, ster. Podpěra, H. P. (sub P. denticulatum). Nové Město n. Met. ad rup. fyllit. Peklo, ster., Šmaeda, H. Š.; Berno, Skryje, ster., cum Anomodon attenuatus et Mnium cuspidatum, Šmaeda, H. Š. (sub P. denticulatum propaguliferum); Tusset, 1000 m, ster., Podpěra, H. P. (sub P. denticulatum). Moravia, Jeseníky, Švýcárna, ster. 1300 m, Podpěra, H. P.; Hokšár, ster., Podpěra, H. P.; Brno, pr. arcem Veveří, ster., Podpěra, H. P.; in conv. Bílý potok, sup. Hluboké, ster. Podpěra, H. P. (sub P. Roeseanum umbrosum); Adamov, in conv. riv., Josefovský, ster., Podpěra, H. P.; in conv. rivuli Kateřinský potok, ster., J. Müller, H. U. B.; ad rup. syenit. in conv. flum. Svitava, inter Adamov et Blansko, ster., Podpěra, H. P.; Rousínov, Vítocický žleb, Podpěra, H. P. (sub P. Roeseanum gracile fo. tenullum) Podp. in sched.; Mor. Krumlov, ad rup. perm., 300 m, ster., Podpěra, H. P.; Carp. occid., ad ped. m. Lysá Hora, pr. Staré Hamry, ster., Podpěra, H. P.; in m. Hostýn, ster., Podpěra, H. P (n.v.).
Plagiothecium roeseanum fo. acuminatum Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 40 (1948); P. cavifolium fo. acuminatum (Jedl.) Z.Iwats., J. Hattorii Bot. Lab. 33: 363. (1970). Type (authentic specimens cited in Jedlička 1961): Austria, Arlingsgraben, ster., Latzel, H. L. Bohemia, Praha, ad rup. lydit., 200 m, ster., Šmarda, H. Š.; Babka pr. Řevnive, 400 m, Bauer, Bryoth. Bohem. no 255, H. U. P., H. Š., H. M. P. (sub P. roeseanum typicum), Mladá Boleslav, in conv. Choboty, cfr., Podpěra, H. P. Moravia, Jeseníky, Dolní Lipová, ster., Latzel, H. L.; in conv. riv. Seifen pr. Vernířovice, 800 m, ster., Podpěra, H. P.; Znajmo, Eisleiten pr. Varanoc, ster., Podpěra, H. P.; Senohrady, ad rup., ster., Podpěra, H. P.; Unčov, ad cataract. Řešovský, 400 m, ster., Podpěra, H. P. Slovakia, Babia Góra, ad lignus putr., ster., Šmerda, H. Š. (sub P. silvaticum longifolium); Bielské Tatry, in conv. Havran, 1100 m, cum Blepharostoma trichophyllum, ster., Šmerda, H. Š (n.v.).
An analysis of original materials of
P. otii (MAK B16360) shows that they are the most different from the previously described groups. Leaves of this specimen are symmetrical or asymmetrical, concave, strongly folded, and strongly serrate at the apex, with quite wide and long cells 100–170 (M 137) × 10–12 µm (M 11) and with very long decurrencies composed of rectangular cells. These features exclude the tested material from the described complex. However, compared to all species of the Northern Hemisphere, this material constitutes a unique set of features. Thus, we propose the resurrection of
P. otii (
Figure 16) as a separate species.
Plagiothecium otii Sakurai, Botanical Magazine (Tokyo) 62: 113, f. 5 (1949); P. cavifolium fo. otii (Sakurai) Z.Iwats., J. Hattori Bot. Lab. 33: 363 (1970). Type: Japan, Prov. Iyo, Mt. Ishizuti, K. Oti, 8 Aug. 1949, herb. K. Sakurai 3388 (holotype: MAK B16360!).
Analysis of the original collection of
P. sakuraii (MAK B609; PC0132597) allows the observation that this specimen is characterized by symmetrical, folded, concave and serrate leaves. However, it differs from the abovementioned taxa in the length and width of cells from the central part of the leaf [87.5–150 (M 119) × 7–10 (M 8.5) µm]. Moreover, detailed analysis of this material showed that the leaf apices are eroded (
Figure 17). These features exclude the tested material from the described complex; thus we propose to resurrect
P. sakuraii.
Plagiothecium sakuraii Reimers, Bot. Jahrb. Syst. 64: 554, 21 f. 3,4 (1931). Lectotype (designated here): Japan, Honsho, Prov. Hitachi, Mt. Tsukuba, an feuchten Felsen, K. Sakurai 609, May 1921, herb. K. Sakurai (PC0132597!), isolectotype: (MAK B609!).
The other analyzed names differ even more from the diagnosis of Hypnum cavifolium and should be excluded not only from the P. cavifolium complex but also (in one case) even from Plagiothecium.
Analysis of original collections of
P. takahashii (MAK B9398) (
Figure 18) showed that the leaves of this specimen are strongly rolled; the cells are short and narrow 25–55 (M 40) × 6–8 (M 7) µm; the stems have multiple layers of thick-walled epidermal cells; pseudoparaphyllia are present on the stems. These features not only exclude the analyzed material from
P. cavifolium sensu lato, but also from the entire genus
Plagiothecium. However, this set of features clearly indicates that this material belongs to
Hygrohypnum luridum (Hedw.) Jenn. Thus, we propose to consider
P. takahashii as a new synonym for this species.
Hygrohypnum luridum (Hedw.) Jenn., Manual Mosses W. Pennsylvania 287 (1913).
Plagiothecium takahashii Sakurai, Bot. Mag. (Tokyo) 51: 79 (1937), syn. nov. Type: Japan, Miyazaki Pref., Mt. Sobo, 24 June 1935, H. Takahashi 108, herb. K. Sakurai 9398 (holotype: MAK B9398!).
Due to the set of features, the next analyzed names also cannot be classified as P. cavifolium sensu lato. Characters such as the asymmetric, broadly ovate leaves with long and wide cells described for P. sylvaticum var. neglectum fo. orthocladum, P. roeseanum var. heterophyllum and P. roeseanum fo. heterophyllum bring to mind the recently reintroduced P. longisetum Lindb. This is also confirmed by quantitative features and figures.
Plagiothecium longisetum Lindb., Contr. Fl. Crypt. As., Acta Soc. Sci. Fenn. 10: 232 (1872). Type: Japan, ad Nikosan ins. Kiusiu, [fertile], 16 Junii 1863, S.O. Lindberg s.n. (lectotype: H-SOL 1563 011!, isolectotype: PC00132572!, S-B160017).
Plagiothecium roeseanum var. heterophyllum Warnst., Krypt.-Fl Brandenburg, Laubm. 814 (1906), syn. nov.; P. roeseanum fo. heterophyllum (Warnst.) Jedl., Spisy Přír. Fak. Masarykovy Univ. 308: 40 (1948). Type: Germany, Brandenburgia, Neurippen, Ruppin, auf Waldboden, Böschungen im “Flössergrunde”, C. Warnstorf; Westprignitz, Forsthaus “Alte Eiche”, auf Waldboden am Standort von Osmunga regalis, Janzen and C. Warnstorf; Wittenberge, Westprignitz, am Grunde eines Baumstammes, “Krauses Brack”, C. Warnstorf; Ratzburg, Buchenwälder, Prahl. Poland, Świnoujście, Weg nach Corswant, R. Ruthe (n.v.).
P. sylvaticum var. neglectum fo. orthocladum Barkman, nom. inval., Buxbaumia 11: 23 (1957), syn. nov. Type: no type was specified.
Whereas P. röseanum var. gracile and P. roeseanum fo. gracile, described as montane plants characterized by, e.g., loose turf with small, distant foliage and with round or almost round decurrency cells. This set of characteristics brings to mind P. denticulatum var. obtusifolium (Turner) Moore or other taxa belonging to the P. denticulatum complex. However, it was not possible to obtain the original materials to verify this hypothesis.
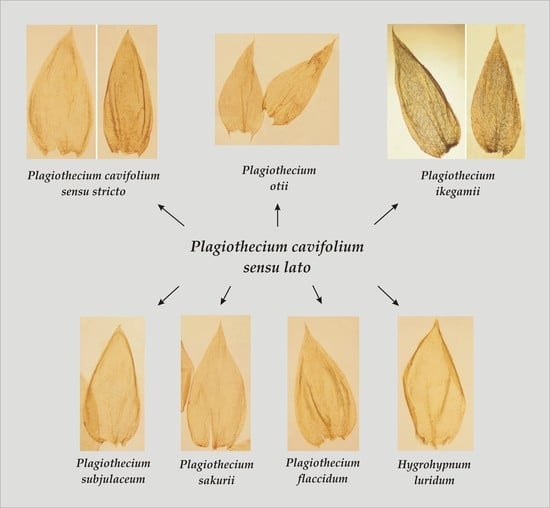
Thus, the conducted research, the analysis of all original materials, available diagnoses and the history of the described taxon show that the Plagiothecium cavifolium complex consists of P. cavifolium (= P. cavifolium sensu stricto), P. flaccidum, P. ikegamii, P. tenue, P. subjulaceum, P. sakuraii and P. otii.
Additionally, the research allowed us to propose P. takahashii as a new synonym for Hygrohypnum luridum, whereas P. sylvaticum var. neglectum fo. orthocladum, P. roeseanum var. heterophyllum and P. roeseanum fo. heterophyllum are proposed as new synonyms for P. longisetum.
The key for species belonging to the P. cavifolium complex
1. Leaves with an eroded apex… P. sakuraii.
1′. Leaves without an eroded apex… 2.
2. Symmetrical and asymmetrical leaves on the stem… P. otii.
2′. Stem leaves symmetrical or separately slightly asymmetrical… 3.
3. Turf julaceous; leaves imbricate, symmetrical, concave; more or less folded… 4.
3′. Turf not julaceous, leaves little or not at all imbricate, flat and not folded… P. tenue.
4. Capsules inclined… 5.
4′. Capsules erect… P. flaccidum.
5. Leaves not serrate… 6.
5′. Leaves serrate… P. ikegamii.
6. The cells from the middle part of the leaf to 101 µm in length… P. cavifolium sensu stricto.
6′. The cells from the middle part of the leaf more than 101 µm in length… P. subjulaceum.