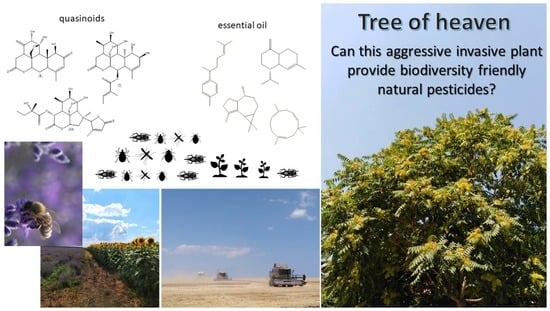
Assessment of the Potential of the Invasive Arboreal Plant Ailanthus altissima (Simaroubaceae) as an Economically Prospective Source of Natural Pesticides
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Ethnobotanical Data about Ailanthus altissima (Mill.) Swingle
3.2. Chemical Constituents of Ailanthus altissima and Extraction Methods
3.3. Essential Oil of Ailanthus altissima: Composition and Extraction Overview
3.4. Quassinoids Extraction, Fractionation, and Isolation Overview
3.5. Biopesticide Potential of Ailanthus altissima and Tests’ Design
3.5.1. Phytotoxicity Assay of Ailanthus altissima
Essential Oil Phytotoxicity
Phytotoxicity of Polar Ailanthus altissima Extracts
3.5.2. Antifungal Activity
3.5.3. Fumigant and Insect Repellent Activity
Essential Oil Fumigant and Insect Repellent Activity
Polar Extracts’ Fumigant and Insect-Repellent Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, B.; Amler, S.; Amler, R.W. Pesticides. Pediatrics 2004, 113 (Suppl. 4), 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- The Editors of Encyclopaedia Britannica. “Pesticide”. Encyclopedia Britannica, 28 July 2022. Available online: https://www.britannica.com/technology/pesticide (accessed on 8 August 2022).
- Alavanja, M.C.; Bonner, M.R. Pesticides and human cancers. Cancer Investig. 2005, 23, 700–711. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Guizzetti, M.; Vitalone, A. Neurotoxicity of pesticides: A brief review. Front. Biosci. 2008, 13, 1240–1249. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Suratman, S.; Edwards, J.W.; Babina, K. Organophosphate pesticides exposure among farmworkers: Pathways and risk of adverse health effects. Rev. Environ. Health 2015, 30, 65–79. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A. Worldwide regulations of standard values of pesticides for human health risk control: A Review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 1, 525–535. [Google Scholar] [CrossRef]
- Landrigan, P.J. Pesticides and Human Reproduction. JAMA Intern. Med. 2018, 178, 26–27. [Google Scholar] [CrossRef]
- Adeyemi, J.A.; Ukwenya, V.O.; Arowolo, O.K.; Olise, C.C. Pesticides-induced Cardiovascular Dysfunctions: Prevalence and Associated Mechanisms. Curr. Hypertens. Rev. 2021, 17, 27–34. [Google Scholar] [CrossRef]
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979, 300, 689–695. [Google Scholar] [CrossRef]
- FAO. Pollinators Vital to Our Food Supply under Threat. 2021. Available online: http://www.fao.org/news/story/en/item/384726/icode/ (accessed on 25 July 2021).
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Brown, M.J.; Paxton, R.J. The conservation of bees: A global perspective. Apidologie 2009, 40, 410–416. [Google Scholar] [CrossRef]
- Potts, S.; Biesmeijer, K.; Bommarco, R.; Breeze, T.; Carvalheiro, L.; Franzén, M.; González-Varo, J.P.; Holzschuh, A.; Kleijn, D.; Klein, A.-M.; et al. Status and Trends of European Pollinators. Key Findings of the STEP Project; Pensoft Publishers: Sofia, Bulgaria, 2015; p. 72. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Carvalheiro, L.G.; Kunin, W.E.; Keil, P.; Aguirre-Gutiérrez, J.; Ellis, W.N.; Fox, R.; Biesmeijer, J.C. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecol. Lett. 2013, 16, 870–878. [Google Scholar] [CrossRef]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Goulson, D.; Frey, H.; Tzinieris, S.; Callaghan, C.; Kerr, J. Call to restrict neonicotinoids. Science 2018, 360, 973. [Google Scholar] [CrossRef]
- Wright, G.A.; Softley, S.; Earnshaw, H. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 2015, 5, 15322. [Google Scholar] [CrossRef]
- Stanley, D.A.; Smith, K.E.; Raine, N.E. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 2015, 5, 16508. [Google Scholar] [CrossRef]
- Stanley, D.A.; Garratt, M.P.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Isaac, N.J.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides?—A brief review. Environ. Int. 2016, 89, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Jiliberto, R.; de Espanés, P.M.; Vázquez, D.P. Pollinator declines and the stability of plant–pollinator networks. Ecosphere 2020, 11, e03069. [Google Scholar] [CrossRef]
- Althaus, S.L.; Berenbaum, M.R.; Jordan, J.; Shalmon, D.A. No buzz for bees: Media coverage of pollinator decline. Proc. Natl. Acad. Sci. USA 2021, 118, e2002552117. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef]
- Boily, M.; Sarrasin, B.; DeBlois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Botías, C.; David, A.; Horwood, J.; Abdul-Sada, A.; Nicholls, E.; Hill, E.; Goulson, D. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 2015, 49, 12731–12740. [Google Scholar] [CrossRef]
- Main, A.R.; Hladik, M.L.; Webb, E.B.; Goyne, K.W.; Mengel, D. Beyond neonicotinoids–Wild pollinators are exposed to a range of pesticides while foraging in agroecosystems. Sci. Total Environ. 2020, 742, 140436. [Google Scholar] [CrossRef]
- English, S.G.; Sandoval-Herrera, N.I.; Bishop, C.A.; Cartwright, M.; Maisonneuve, F.; Elliott, J.E.; Welch, K.C. Neonicotinoid pesticides exert metabolic effects on avian pollinators. Sci. Rep. 2021, 11, 2914. [Google Scholar] [CrossRef]
- Chan, D.S.W.; Raine, N.E. Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Sci. Rep. 2021, 11, 4241. [Google Scholar] [CrossRef] [PubMed]
- Bloom, E.H.; Wood, T.J.; Hung, K.L.J.; Ternest, J.J.; Ingwell, L.L.; Goodell, K.; Szendrei, Z. Synergism between local- and landscape-level pesticides reduces wild bee floral visitation in pollinator-dependent crops. J. Appl. Ecol. 2021, 58, 1187–1198. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Abraham, J.; Benhotons, G.S.; Krampah, I.; Tagba, J.; Amissah, C.; Abraham, J.D. Commercially formulated glyphosate can kill non-target pollinator bees under laboratory conditions. Entomol. Exp. Appl. 2018, 166, 695–702. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Balbuena, M.S.; Chaves, F.; Gora, J.; Menzel, R.; Farina, W.M. Sleep in honey bees is affected by the herbicide glyphosate. Sci. Rep. 2020, 10, 10516. [Google Scholar] [CrossRef]
- Vázquez, D.E.; Ilina, N.; Pagano, E.A.; Zavala, J.A.; Farina, W.M. Glyphosate affects the larval development of honey bees depending on the susceptibility of colonies. PLoS ONE 2018, 13, e0205074. [Google Scholar] [CrossRef]
- Haas, J.; Nauen, R. Pesticide risk assessment at the molecular level using honey bee cytochrome P450 enzymes: A complementary approach. Environ. Int. 2021, 147, 106372. [Google Scholar] [CrossRef]
- Battisti, L.; Potrich, M.; Sampaio, A.R.; de Castilhos Ghisi, N.; Costa-Maia, F.M.; Abati, R.; Sofia, S.H. Is glyphosate toxic to bees? A meta-analytical review. Sci. Total Environ. 2021, 767, 145397. [Google Scholar] [CrossRef]
- Hipólito, J.; Coutinho, J.; Mahlmann, T.; Santana, T.B.R.; Magnusson, W.E. Legislation and pollination: Recommendations for policymakers and scientists. Perspect. Ecol. Conserv. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Gemmill-Herren, B.; Garibaldi, L.A.; Kremen, C.; Ngo, H.T. Building effective policies to conserve pollinators: Translating knowledge into policy. Curr. Opin. Insect. Sci. 2021, 46, 64–71. [Google Scholar] [CrossRef]
- Shaaya, E.; Ravid, U.; Paster, N.; Juven, B.; Zisman, U.; Pissarev, V. Fumigant toxicity of essential oils against four major stored-product insects. J. Chem. Ecol. 1991, 17, 499–504. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. J. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Suthisut, D.; Fields, P.G.; Chandrapatya, A. Fumigant toxicity of essential oils from three Thai plants (Zingiberaceae) and their major compounds against Sitophilus zeamais, Tribolium castaneum and two parasitoids. J. Stored Prod. Res. 2011, 47, 222–230. [Google Scholar] [CrossRef]
- Polatoğlu, K.; Karakoç, Ö.C.; Gören, N. Phytotoxic, DPPH scavenging, insecticidal activities and essential oil composition of Achillea vermicularis, A. teretifolia and proposed chemotypes of A. biebersteinii (Asteraceae). Ind. Crops Prod. 2013, 51, 35–45. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Peñalver, D.; Santana-Méridas, O. Antifeedant effects of common terpenes from Mediterranean aromatic plants on Leptinotarsa decemlineata. J. Plant Nutr. Soil Sci. 2017, 17, 475–485. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Haig, T.J.; Haig, T.J.; Seal, A.N.; Pratley, J.E.; An, M.; Wu, H. Lavender as a source of novel plant compounds for the development of a natural herbicide. J. Chem. Ecol. 2009, 35, 1129–1136. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef]
- Wright, C.; Chhetri, B.K.; Setzer, W.N. Chemical composition and phytotoxicity of the essential oil of Encelia farinosa growing in the Sonoran Desert. Am. J. Essent. Oil. Nat. Prod. 2013, 1, 18–22. [Google Scholar]
- De Feo, V.; Mancini, E.; Voto, E.; Curini, M.; Digilio, M.C. Bioassay-oriented isolation of an insecticide from Ailanthus altissima. J. Plant Interact. 2009, 4, 119–123. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.; Yang, T.; Wang, H.; Liao, H.; Liang, D. Quassinoids with insecticidal activity against diaphorina citri kuwayama and neuroprotective activities from Picrasma quassioides. J. Agric. Food Chem. 2019, 68, 117–127. [Google Scholar] [CrossRef]
- Fang, X.; Di, Y.T.; Zhang, Y.; Xu, Z.P.; Lu, Y.; Chen, Q.Q.; Zheng, Q.T.; Hao, X.J. Unprecedented quassinoids with promising biological activity from Harrisonia perforata. Angew. Chem. Int. Ed. 2015, 54, 5592–5595. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- DAISIE. Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Petrova, A.; Vladimirov, V.; Georgiev, V. Invasive Alien Plant Species in Bulgaria; Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2012. (In Bulgarian) [Google Scholar]
- Zahariev, D. Invasive plant species along the major rivers in Strandzha Natural Park. In Proceedings of the Seminar of Ecology—2014, Sofia, Bulgaria, 24–25 April 2014; pp. 148–158. [Google Scholar]
- Monaco, A. European Guidelines on Protected Areas and Invasive Alien Species; Council of Europe: Rome, Italy, 2014. [Google Scholar]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Global Invasive Species Database. Species Profile: Ailanthus altissima. 2019. Available online: http://www.iucngisd.org/gisd/species.php?sc=319 (accessed on 25 July 2022).
- Domina, G. Invasive Aliens in Italy: Enumeration, History, Biology and Their Impact. In Invasive Alien Species: Observations and Issues from Around the World; Pullaiah, T., Ielmini, M.R., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 3, pp. 190–214. [Google Scholar] [CrossRef]
- Demeter, A.; Saláta, D.; Tormáné Kovács, E.; Szirmai, O.; Trenyik, P.; Meinhardt, S.; Czóbel, S. Effects of the Invasive Tree Species Ailanthus altissima on the Floral Diversity and Soil Properties in the Pannonian Region. Land 2021, 10, 1155. [Google Scholar] [CrossRef]
- Motti, R.; Zotti, M.; Bonanomi, G.; Cozzolino, A.; Stinca, A.; Migliozzi, A. Climatic and anthropogenic factors affect Ailanthus altissima invasion in a Mediterranean region. Plant Ecol. 2021, 222, 1347–1359. [Google Scholar] [CrossRef]
- Terzi, M.; Fontaneto, D.; Casella, F. Effects of Ailanthus altissima Invasion and Removal on High-Biodiversity Mediterranean Grasslands. Environ. Manag. 2021, 68, 914–927. [Google Scholar] [CrossRef]
- Pedersini, C.; Bergamin, M.; Aroulmoji, V.; Baldini, S.; Picchio, R.; Pesce, P.G.; Ballarin, L.; Murano, E. Herbicide Activity of Extracts from Ailanthus altissima (Simaroubaceae). Nat. Prod. Commun. 2011, 6, 593–596. [Google Scholar] [CrossRef]
- Kubota, K.; Fukamiya, N.; Hamada, T.; Okano, M.; Tagahara, K.; Lee, K.H. Two new quassinoids, ailantinols A and B, and related compounds from Ailanthus altissima. J. Nat. Prod. 1996, 59, 683–686. [Google Scholar] [CrossRef]
- Kubota, K.; Fukamiya, N.; Okano, M.; Tagahara, K.; Lee, K.H. Two new quassinoids, ailantinols C and D, from Ailanthus altissima. Bull. Chem. Soc. Jpn. 1996, 69, 3613–3617. [Google Scholar] [CrossRef]
- Tamura, S.; Fukamiya, N.; Okano, M.; Koyama, J.; Koike, K.; Tokuda, H.; Nishino, H. Three new quassinoids, ailantinol E, F, and G, from Ailanthus altissima. Chem. Pharm. Bull. 2003, 51, 385–389. [Google Scholar] [CrossRef]
- Takeya, K.; Kobata, H.; Ozeki, A.; Morita, H.; Itokawa, H. A new quassinoid from Ailanthus vilmoriniana. J. Nat. Prod. 1997, 60, 642–644. [Google Scholar] [CrossRef]
- Joshi, B.C.; Pandey, A.; Sharma, R.P.; Khare, A. Quassinoids from Ailanthus excelsa. Phytochemistry 2003, 62, 579–584. [Google Scholar] [CrossRef]
- Manimaran, V.; Suganthy, M.; Balasubramanian, A.; Kumar, P.P. Management of tea mosquito bug, Helopeltis antonii Signoret infesting Ailanthus excelsa Roxb. J. Entomol. Zool. Stud. 2019, 7, 620–623. [Google Scholar]
- Karalija, E.; Dahija, S.; Parić, A.; Zeljković, S.Ć. Phytotoxic potential of selected essential oils against Ailanthus altissima (Mill.) Swingle, an invasive tree. Sust. Chem. Pharm. 2020, 15, 100219. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Ma, S.; Zhao, Q.; Wu, J.; Duan, L.; Wang, S. Traditional uses, phytochemistry, and pharmacology of Ailanthus altissima (Mill.) Swingle bark: A comprehensive review. J. Ethnopharmacol. 2021, 275, 114121. [Google Scholar] [CrossRef]
- Lü, J.H.; He, Y.Q. Fumigant toxicity of Ailanthus altissima Swingle, Atractylodes lancea (Thunb.) DC. and Elsholtzia stauntonii Benth extracts on three major stored-grain insects. Ind. Crops Prod. 2010, 32, 681–683. [Google Scholar] [CrossRef]
- Ohmoto, T.; Koike, K.; Sakamoto, Y. Studies on the constituents of A. altissima Swingle II. The alkaloid constituent. Chem. Pharm. Bull. 1981, 29, 390–395. [Google Scholar] [CrossRef]
- Ohmoto, T.; Koike, K. Studies on the constituents of A. altissima Swingle III. The alkaloid constituents. Chem. Pharm. Bull. 1984, 32, 170–173. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I. Volatile Constituents from the Leaves of Young and Old Ailanthus altissima (Mili.) Swingle Tree. Croat. Chem. Acta 2002, 75, 189–197. [Google Scholar]
- Kozuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Kochmarov, V. Ailanthus altissima (Mill.) Swingle—A terrible invasive pest in Bulgaria or potential useful medicinal plant? Bothalia 2014, 44, 213–230. [Google Scholar]
- Zhelev, I.; Georgiev, K.; Dimitrova-Dyulgerova, I. Carotenoid profile of Ailanthus altissima stem bark, in-vitro antioxidant and antineoplastic activities. World J. Pharm. Res. 2016, 5, 1816. [Google Scholar]
- Cho, S.K.; Jeong, M.; Jang, D.S.; Choi, J.H. Anti-inflammatory Effects of Canthin-6-one Alkaloids from Ailanthus altissima. Planta Med. 2018, 50, 527–535. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Rusak, G.; Eloff, J.N. Phenolic composition of leaf extracts of Ailanthus altissima (Simaroubaceae) with antibacterial and antifungal activity equivalent to standard antibiotics. Nat. Prod. Commun. 2017, 12, 1934578X1701201021. [Google Scholar] [CrossRef]
- Du, Y.Q.; Yan, Z.Y.; Shi, S.C.; Hou, Z.L.; Huang, X.X.; Song, S.J. Benzoic acid derivatives from the root barks of Ailanthus altissima. J. Asian Nat. Prod. Res. 2021, 23, 103–109. [Google Scholar] [CrossRef]
- Du, Y.Q.; Yan, Z.Y.; Chen, J.J.; Wang, X.B.; Huang, X.X.; Song, S.J. The identification of phenylpropanoids isolated from the root bark of Ailanthus altissima (Mill.) Swingle. Nat. Prod. Res. 2021, 35, 1139–1146. [Google Scholar] [CrossRef]
- Du, Y.Q.; Bai, M.; Yu, X.Q.; Lv, T.M.; Lin, B.; Huang, X.X.; Song, S.J. Quassinoids from the Root Barks of Ailanthus altissima: Isolation, Configurational Assignment, and Cytotoxic Activities. Chin. J. Chem. 2021, 39, 879–886. [Google Scholar] [CrossRef]
- Wang, C.M.; Li, H.F.; Wang, X.K.; Li, W.G.; Su, Q.; Xiao, X.; Zhang, C.H. Ailanthus altissima-derived ailanthone enhances gastric cancer cell apoptosis by inducing the repression of base excision repair by downregulating p23 Expression. Int. J. Biol. Sci. 2021, 17, 2811. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.K.; Lin, B.; Du, Y.Q.; Li, C.; Yu, X.Q.; Xue, X.B.; Huang, X.X. Monoterpenoid coumarins and monoterpenoid phenylpropanoids from the root bark of Ailanthus altissima. New J. Chem. 2021, 45, 1100–1108. [Google Scholar] [CrossRef]
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gallardo, E. Insights into the Bioactivities and Chemical Analysis of Ailanthus altissima (Mill.) Swingle. Appl. Sci. 2021, 11, 11331. [Google Scholar] [CrossRef]
- Bray, D.H.; Boardman, P.; ONeill, M.J.; Chan, K.L.; Phillipson, J.D.; Warhurst, D.C.; Suffness, M. Plants as a source of antimalarial drugs 5. Activities of Ailanthus altissima stem constituents and of some related quassinoids. Phytother. Res. 1987, 1, 22–24. [Google Scholar] [CrossRef]
- Okunade, A.L.; Bikoff, R.E.; Casper, S.J.; Oksman, A.; Goldberg, D.E.; Lewis, W.H. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae). Phytother. Res. 2003, 17, 675–677. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Zhang, Z. Quantitative proteomics reveals the antifungal effect of canthin-6-one isolated from Ailanthus altissima against Fusarium oxysporum f. sp. cucumerinum in vitro. PLoS ONE 2021, 16, e0250712. [Google Scholar] [CrossRef]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. S. Afr. J. Bot. 2013, 87, 164–174. [Google Scholar] [CrossRef]
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical Composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) Swingle cultivated in Tunisia. Chem. Biodivers. 2014, 11, 1216–1227. [Google Scholar] [CrossRef]
- Kozuharova, E.; Benbassat, N.; Berkov, S.; Ionkova, I. Ailanthus altissima and Amorpha fruticosa—Invasive arboreal alien plants as cheap sources of valuable essential oils. Pharmacia 2020, 67, 71. [Google Scholar] [CrossRef]
- Lü, J.; Wu, S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010, 4, 154–157. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Wang, K.; Xu, J.; Zhao, J.; Shan, T.; Luo, C. Secondary metabolites with antinematodal activity from higher plants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 67–114. [Google Scholar] [CrossRef]
- He, Q.; Xiao, H.; Li, J.; Liu, Y.; Jia, M.; Wang, F.; Zhang, Y.; Wang, W.; Wang, S. Fingerprint analysis and pharmacological evaluation of Ailanthus altissima. Int. J. Mol. Med. 2018, 41, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Mao, X.X.; Zhou, J.; Zhang, M.L.; Wu, Y.B.; Huo, C.H.; Gu, Y.C. Antitumor activities of six quassinoids from Ailanthus altissima. Chem. Nat. Compd. 2017, 53, 28–32. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.J.; Su, C.; Zhang, D.M.; Xu, L.P.; He, R.R.; Ye, W.C. Cytotoxic quassinoids from Ailanthus altissima. Bioorg. Med. Chem. Lett. 2013, 23, 654–657. [Google Scholar] [CrossRef]
- Naora, H.; Ishibashi, M.; Furuno, T.; Tsuyuki, T.; Murae, T.; Hirota, H.; Takahashi, T.; Itai, A.; Iitaka, Y. Structure determination of bitter principles in Ailanthus altissima. Structure of shinjulactone A and revised structure of ailanthone. Bull. Chem. Soc. Jpn. 1983, 56, 3694–3698. [Google Scholar] [CrossRef]
- Tamura, S.; Fukamiya, N.; Okano, M.; Koike, K. A new quassinoid, ailantinol H from Ailanthus altissima. Nat. Prod. Res. 2006, 20, 1105–1109. [Google Scholar] [CrossRef]
- Ishibashi, M.; Tsuyuki, T.; Murae, T.; Hirota, H.; Takahashi, T.; Itai, A.; Iitaka, Y. Constituents of the Root Bark of Ailanthus altissima S WINGLE. Isolation and X-Ray Crystal Structures of Shinjudilactone and Shinjulactone C and Conversion of Ailanthone into Shinjudilactone. Bull. Chem. Soc. Jpn. 1983, 56, 3683–3693. [Google Scholar] [CrossRef]
- Yoshimura, S.; Ishibashi, M.; Tsuyuki, T.; Takahashi, T.; Matsushita, K. Constituents of seeds of Ailanthus altissima Swingle. Isolation and structures of shinjuglycosides A, B, C, and D. Bull. Chem. Soc. Jpn. 1984, 57, 2496–2501. [Google Scholar] [CrossRef]
- Niimi, Y.; Tsuyuki, T.; Takahashi, T.; Matsushita, K. Bitter principles of Ailanthus altissima Swingle. Structure determination of shinjuglycosides E and F. Chem. Pharm. Bull. 1987, 35, 4302–4306. [Google Scholar] [CrossRef]
- Furuno, T.; Ishibashi, M.; Naora, H.; Murae, T.; Hirota, H.; Tsuyuki, T.; Iitaka, Y. Structure determination of bitter principles of Ailanthus altissima. Structures of shinjulactones B, D, and E. Bull. Chem. Soc. Jpn. 1984, 57, 2484–2489. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yoshimura, S.; Tsuyuki, T.; Takahashi, T.; Itai, A.; Iitaka, Y. Structure determination of bitter principles of Ailanthus altissima. Structures of shinjulactones F, I, J, and K. Bull. Chem. Soc. Jpn. 1984, 57, 2885–2892. [Google Scholar] [CrossRef]
- Ishibashi, M.; Yoshimura, S.; Tsuyuki, T.; Takahashi, T.; Matsushita, K. Shinjulactones G and H, new bitter principles of Ailanthus altissima Swingle. Bull. Chem. Soc. Jpn. 1984, 57, 2013–2014. [Google Scholar] [CrossRef]
- Ishibashi, M.; Tsuyuki, T.; Takahashi, T. Structure determination of a new bitter principle, shinjulactone L, from Ailanthus altissima. Bull. Chem. Soc. Jpn. 1985, 58, 2723–2724. [Google Scholar] [CrossRef]
- Niimi, Y.; Tsuyuki, T.; Takahashi, T.; Matsushita, K. Structure determination of shinjulactones M and N, new bitter principles from Ailanthus altissima Swingle. Bull. Chem. Soc. Jpn. 1986, 59, 1638–1640. [Google Scholar] [CrossRef]
- Yang, X.L.; Yuan, Y.L.; Zhang, D.M.; Li, F.; Ye, W.C. Shinjulactone O, a new quassinoid from the root bark of Ailanthus altissima. Nat. Prod. Res. 2014, 28, 1432–1437. [Google Scholar] [CrossRef]
- Tan, Q.W.; Ni, J.C.; Zheng, L.P.; Fang, P.H.; Shi, J.T.; Chen, Q.J. Anti-Tobacco mosaic virus quassinoids from Ailanthus altissima (Mill.) Swingle. J. Agric. Food Chem. 2018, 66, 7347–7357. [Google Scholar] [CrossRef]
- Tsao, R.; Romanchuk, F.E.; Peterson, C.J.; Coats, J.R. Plant growth regulatory effect and insecticidal activity of extracts of tree of Heaven (Ailanthus altissima L.). BMC Ecol. 2002, 2, 1. Available online: https://bmcecol.biomedcentral.com/articles/10.1186/1472-6785-2-1 (accessed on 25 July 2022).
- Quintana, N.; El Kassis, E.G.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic compounds from roots of Centaurea diffusa Lam. Plant Signal. Behav. 2009, 4, 9–14. [Google Scholar] [CrossRef]
- De Martino, L.; Formisano, C.; Mancini, E.; Feo, V.D.; Piozzi, F.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils from four Teucrium species. Nat. Prod. Commun. 2010, 5, 1969–1976. [Google Scholar] [CrossRef]
- Szabó, L. Juglone index—A possibility for expressing allelopathic potential of plant taxa with various life strategies. Acta Bot. Hung. 1999, 42, 295–305. [Google Scholar]
- Csiszár, Á. Allelopathic effects of invasive woody plant species in Hungary. Acta Silv. Lignaria Hung. 2009, 5, 9–17. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1066.4899&rep=rep1&type=pdf (accessed on 25 July 2022).
- Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Süle, P.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Allelopathic potential of some invasive plant species occurring in Hungary. Allelopath. J. 2013, 31, 309–318. [Google Scholar]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Heisy, R. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- Bostan, C.; Borlea, F.; Mihoc, C.; Selesan, M. Ailanthus altissima species invasion on biodiversity caused by potential allelopathy. J. Agric. Sci. 2014, 46, 95–103. Available online: http://cormoran.portiledefier.ro/wp-content/uploads/2013/02/bostan_cristian_1.pdf (accessed on 25 July 2022).
- Sladonja, B.; Pohulja, D.; Sušek, M.; Dudaš, S. Herbicidal effect of Ailanthus altissima leaves water extracts on Medicago sativa seeds germination. In Book of Abstracts of the 3rd Conference with International Participation Conference VIVUS; Biotechnical Centre Naklo: Naklo, Slovenia, 2014; pp. 476–481. Available online: http://civ.iptpo.hr/wp-content/uploads/publikacije/Znanstveni%20rad%20u%20zborniku%20skupa_VIVUS_2014.pdf (accessed on 25 July 2022).
- Heisey, R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 1996, 83, 192–200. [Google Scholar] [CrossRef]
- Casinovi, C.G.; Ceccherelli, P.; Fardella, G.; Grandolini, G. Isolation and structure of a quassinoid from Ailanthus glandulosa. Phytochemistry 1983, 22, 2871–2873. [Google Scholar] [CrossRef]
- Lin, L.-J.; Peiser, G.; Ying, B.-P.; Mathias, K.; Karasina, F.; Wang, Z.; Itatani, J.; Green, L.; Hwang, Y.-S. Identification of plant growth inhibitory principles in Ailanthus altissima and Castela tortuosa. J. Agric. Food Chem. 1995, 43, 1706–1711. [Google Scholar] [CrossRef]
- De Feo, V.; De Martino, L.; Quaranta, E.; Pizza, C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle). J. Agric. Food Chem. 2003, 51, 1177–1180. [Google Scholar] [CrossRef]
- De Feo, V.; Martino, L.D.; Santoro, A.; Leone, A.; Pizza, C.; Franceschelli, S.; Pascale, M. Antiproliferative effects of tree-of-heaven (Ailanthus altissima Swingle). Phytother. Res. 2005, 19, 226–230. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Krutovsky, K.V.; Shestibratov, K.A. Fell Upas Sits, the Hydra-Tree of Death†, or the Phytotoxicity of Trees. Molecules 2019, 24, 1636. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plant Res. 2008, 2, 98–110. [Google Scholar] [CrossRef]
- Heisey, R.M.; Heisey, T.K. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 2003, 256, 85–99. [Google Scholar] [CrossRef]
- Anonymous. National Center for Biotechnology Information. PubChem Database. Ailanthone, CID=72965; 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ailanthone (accessed on 1 January 2022).
- Balkan, B.; Balkan, S.; Aydoğdu, H.; Özcan, Ö. Antifungal activities of Ailanthus altissima Swingle and Juglans regia L. leaves against some cereal fungi. J. Appl. Environ. Biol. Sci. 2014, 8, 76–79. [Google Scholar]
- Jabeen, K.; Asad, S.; Zakria, M. Antifungal Evaluation and Phytochemical Identification of Selected Botanicals against Ceratocystis manginecans Causing Mango Sudden Death. J. Plant Pathol. Microbiol. 2018, 9, 465. [Google Scholar] [CrossRef]
- Joshi, B.C.; Pandey, A.; Chaurasia, L.; Pal, M.; Sharma, R.P.; Khare, A. Antifungal activity of the stem bark of Ailanthus excelsa. Fitoterapia 2003, 74, 689–691. [Google Scholar] [CrossRef]
- Chen, J.J.; Bai, W.; Lu, Y.B.; Feng, Z.Y.; Gao, K.; Yue, J.M. Quassinoids with Inhibitory Activities against Plant Fungal Pathogens from Picrasma javanica. J. Nat. Prod. 2021, 84, 2111–2120. [Google Scholar] [CrossRef]
- Lü, J. The insecticidal activities of Ailanthus altissima extracts on several kinds of important stored-grain insects. Grain Storage 2007, 36, 17–20. [Google Scholar]
- Lü, J.H.; Lu, Y.J.; Hu, Y.Y. Controlling effects of three plant essential oils on Liposcelis paeta. J. Henan Agric. Sci. 2006, 5, 18. [Google Scholar]
- Lü, J.H.; Shi, Y.L. The bioactivitiy of essential oil from Ailanthus altissima Swingle (Sapindales: Simaroubaceae) bark on Lasioderma serricorne (Fabricius) (Coleoptera: Anobiidae). Adv. Mater. Res. 2012, 365, 428–432. [Google Scholar] [CrossRef]
- Wei, J.; Kang, L. Roles of (Z)-3-hexenol in plant-insect interactions. Plant Signal. Behav. 2011, 6, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.M.; Salter, S.S.; Walters, S. Caryophyllene: An attractant for the green lacewing. Environ. Entomol. 1979, 8, 1123–1125. [Google Scholar] [CrossRef]
- Goulson, D. The Garden Jungle: Or Gardening to Save the Planet; Random House: New York, NY, USA, 2019; p. 261. [Google Scholar]
- Gu, X.; Fang, C.; Yang, G.; Xie, Y.; Nong, X.; Zhu, J.; Wang, S.; Peng, X.; Yan, Q. Acaricidal properties of an Ailanthus altissima bark extract against Psoroptes cuniculi and Sarcoptes scabiei var. cuniculi in vitro. Exp. Appl. Acarol. 2014, 62, 225–232. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E, E)-2, 4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, L.; Zitti, S.; Taffetani, F. Ethnobotanical uses in the Ancona district (Marche region, Central Italy). J. Ethnobiol. Ethnomed. 2019, 15, 9. [Google Scholar] [CrossRef]
- Wagner, R.L.; Card, J.A. Ailanthus altissima aqueous extract deters Spodoptera frugiperda oviposition. Gt. Lakes Entomol. 2020, 53, 11. Available online: https://scholar.valpo.edu/tgle/vol53/iss1/11 (accessed on 25 July 2022).
- Wagner, L.R.; Leach, E.M.; Wallace, J.R. Leaf Extract from Ailanthus altissima negatively impacts life history aspects in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Kansas Entomol. Soc. 2021, 93, 140–152. [Google Scholar] [CrossRef]
- Souza, J.R.; Carvalho, G.A.; Moura, A.P.; Couto, M.H.; Maia, J.B. Impact of insecticides used to control Spodoptera frugiperda (JE Smith) in corn on survival, sex ratio, and reproduction of Trichogramma pretiosum Riley offspring. Chil. J. Agric. Res. 2013, 73, 122–127. [Google Scholar] [CrossRef]
- Lu, J.-H.L.; Lu, Y.J.; Tan, Y.B.; Liu, J.J.; Zhong, J.F. The controlling effects of plant extracts on Oryzaephilus surinamensis (Linnaeus). J. Henan Uni. Tech. 2006, 3, 17–20. [Google Scholar]
- Chermenskaya, T.D.; Stepanycheva, E.A.; Shchenikova, A.V.; Chakaeva, A.S. Insectoacaricidal and deterrent activities of extracts of Kyrgyzstan plants against three agricultural pests. Ind. Crops Prod. 2010, 32, 157–163. [Google Scholar] [CrossRef]
- Stepanycheva, E.A.; Chermenskya, T.D.; Chakaeva, A.S. Effect of biologically active substances of Ailanthus altissima Mill. Swingle)(Simarubaceae) on spider mite Tetranychus urticae Koch (Akari: Tetranychidae). Agric. Chem. 2011, 4, 52–59. (In Russian) [Google Scholar]
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of quassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 993–998. [Google Scholar] [CrossRef]
- Pavela, R.; Zabka, M.; Tylova, T.; Kresinova, Z. Insecticidal activity of compounds from Ailanthus altissima against Spodoptera littoralis larvae. Pak. J. Agric. Sci. 2014, 51, 101–112. Available online: https://pakjas.com.pk/papers/2248.pdf (accessed on 25 July 2022).
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2011, 28, 1087–1117. [Google Scholar] [CrossRef]
- Slave, J. Effects of Calcium hydroxide and Quassia extract on Honey bees (Apis mellifera). In Proceedings of the 18th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 19–21 February 2018; Foerdergemeinschaft Oekologischer Obstbau e.V. (FOEKO): Weinsberg, Germany, 2018; pp. 247–248. [Google Scholar]
- Yang, K.; Wen, X.; Ren, Y.; Wen, J. Control of Eucryptorrhynchus scrobiculatus (Coleoptera: Cuculionidae), a major pest of Ailanthus altissima (Sapindales: Simaroubaceae), using a modified square trap net. J. Econ. Entomol. 2018, 111, 1760–1767. [Google Scholar] [CrossRef]
- Todorova, T.; Boyadzhiev, K.; Shkondrov, A.; Parvanova, P.; Dimitrova, M.; Ionkova, I.; Kozuharova, E.; Chankova, S. Screening of Amorpha fruticosa and Ailanthus altissima extracts for genotoxicity/antigenotoxicity, mutagenicity/antimutagenicity and carcinogenicity/anticarcinogenicity. BioRisk 2022, 17, 201–212. [Google Scholar] [CrossRef]


| Compound | CAS Registry Number | Plant Material | Contents or Obtained Amount mg/g Dry Weight | Ref. | |
|---|---|---|---|---|---|
| 1 | 2-dihydroailanthone | not assigned | Bark | 0.027 | [103] |
| 2 | 6α-tigloyloxychaparrin | 75144-71-7 | Root bark | 0.003 | [104] |
| 3 | 6α-tigloyloxychaparrinone | 69423-70-7 | Seedling | 0.017 | [104] |
| 4 | 11-acetylamarolide | 29913-88-0 | Bark, seed | 0.018 | [104] |
| 5 | 12-dihydroisoailanthone | n. a | Bark | 0.080 | [103] |
| 6 | 13,18-dehydroglaucarubinone | 68703-94-6 | Root bark | 0.124 | [104] |
| 7 | Ailanthone | 981-15-7 | Root, seed, leaves | 0.003–0.05 | [103,105] |
| 8 | Ailantinol A | 176181-83-2 | Aerial parts | 0.007 | [72] |
| 9 | Ailantinol B | 177794-39-7 | Stem bark | 0.002 | [72] |
| 10 | Ailantinol C | n. a | Stem bark | 0.002 | [73] |
| 11 | Ailantinol D | n. a | Stem bark | 0.0005 | [73] |
| 12 | Ailantinol E | n. a | Root bark | 0.0004 | [74] |
| 13 | Ailantinol F | n. a | Aerial parts | 0.0004 | [74] |
| 14 | Ailantinol G | n. a | Aerial parts | 0.0007 | [74] |
| 15 | Ailantinol H | n. a | Aerial parts | 0.0002 | [106] |
| 16 | Altissinol A | n. a | Bark | 0.001 | [104] |
| 17 | Altissinol B | n. a | Bark | 0.003 | [104] |
| 18 | Amarolide | 29913-86-8 | Bark, seed | 0.001 | [104] |
| 19 | Chaparrinone | 22611-34-3 | Root bark | 0.002 | [104] |
| 20 | Chaparrolide | 33512-38-8 | Bark | 0.003 | [104] |
| 21 | Δ13−18-dehydroglaucarubolone | n. a | Seed | 0.0002 | [72,104] |
| 22 | Glaucarubin | 1448-23-3 | Stem bark | 0.003 | [104] |
| 23 | Glaucarubinone | 1259-86-5 | Seed | n. a | - |
| 24 | Glaucarubol | 1448-22-2 | Stem bark | n. a | - |
| 25 | Isoailanthone | n. a | Root bark | 0.0002 | [103] |
| 26 | Shinjudilactone | 80180-30-9 | Seed | 0.003 | [107] |
| 27 | Shinjuglycoside A | n. a | Seed | 0.012 | [108] |
| 28 | Shinjuglycoside B | n. a | Seed | 0.044 | [108] |
| 29 | Shinjuglycoside C | n. a | Seed | 0.005 | [108] |
| 30 | Shinjuglycoside D | n. a | Seed | 0.002 | [108] |
| 31 | Shinjuglycoside E | 112667-45-5 | Root bark | 0.0002 | [109] |
| 32 | Shinjuglycoside F | 112667-46-6 | Root bark | 0.00005 | [109] |
| 33 | Shinjulactone A | 89353-91-3 | Seed | 0.002 | [105] |
| 34 | Shinjulactone B | 80648-28-8 | Aerial parts | 0.001–0.004 | [110] |
| 35 | Shinjulactone C | 82470-74-4 | Root bark | 0.001 | [107] |
| 36 | Shinjulactone F | n. a | Root bark | 0.003 | [111] |
| 37 | Shinjulactone G | n. a | Root bark | 0.0003 | [112] |
| 38 | Shinjulactone H | n. a | Root bark | 0.001 | [112] |
| 39 | Shinjulactone I | n. a | Root bark | 0.0002 | [111] |
| 40 | Shinjulactone J | n. a | Root bark | 0.0001 | [111] |
| 41 | Shinjulactone K | 94451-22-6 | Root bark | 0.0005 | [111] |
| 42 | Shinjulactone L | n. a | Root bark | 0.0005 | [113] |
| 43 | Shinjulactone M | n. a | Root bark | 0.0005 | [114] |
| 44 | Shinjulactone N | n. a | Root bark | 0.0002 | [114] |
| 45 | Shinjulactone O | n. a | Root bark | 0.001 | [115] |
| 46 | Chuglycoside A | n. a | Seed (samara) | 0.003 | [116] |
| 47 | Chuglycoside B | n. a | Seed (samara) | 0.014 | [116] |
| 48 | Chuglycoside C | n. a | Seed (samara) | 0.024 | [116] |
| 49 | Chuglycoside D | n. a | Seed (samara) | 0.001 | [116] |
| 50 | Chuglycoside E | n. a | Seed (samara) | 0.145 | [116] |
| 51 | Chuglycoside F | n. a | Seed (samara) | 0.002 | [116] |
| 52 | Chuglycoside G | n. a | Seed (samara) | 0.001 | [116] |
| 53 | Chuglycoside H | n. a | Seed (samara) | 0.0005 | [116] |
| 54 | Chuglycoside I | n. a | Seed (samara) | 0.032 | [116] |
| 55 | Chouchunlactone A | n. a | Root bark | 0.0001 | [90] |
| 56 | Chouchunlactone B | n. a | Root bark | 0.0003 | [90] |
| 57 | Chouchunlactone C | n. a | Root bark | 0.0007 | [90] |
| 58 | Chouchunlactone D | n. a | Root bark | 0.0002 | [90] |
| 59 | Chouchunlactone E | n. a | Root bark | 0.0002 | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozuharova, E.; Pasdaran, A.; Al Tawaha, A.R.; Todorova, T.; Naychov, Z.; Ionkova, I. Assessment of the Potential of the Invasive Arboreal Plant Ailanthus altissima (Simaroubaceae) as an Economically Prospective Source of Natural Pesticides. Diversity 2022, 14, 680. https://doi.org/10.3390/d14080680
Kozuharova E, Pasdaran A, Al Tawaha AR, Todorova T, Naychov Z, Ionkova I. Assessment of the Potential of the Invasive Arboreal Plant Ailanthus altissima (Simaroubaceae) as an Economically Prospective Source of Natural Pesticides. Diversity. 2022; 14(8):680. https://doi.org/10.3390/d14080680
Chicago/Turabian StyleKozuharova, Ekaterina, Ardalan Pasdaran, Abdel Rahman Al Tawaha, Teodora Todorova, Zheko Naychov, and Iliana Ionkova. 2022. "Assessment of the Potential of the Invasive Arboreal Plant Ailanthus altissima (Simaroubaceae) as an Economically Prospective Source of Natural Pesticides" Diversity 14, no. 8: 680. https://doi.org/10.3390/d14080680
APA StyleKozuharova, E., Pasdaran, A., Al Tawaha, A. R., Todorova, T., Naychov, Z., & Ionkova, I. (2022). Assessment of the Potential of the Invasive Arboreal Plant Ailanthus altissima (Simaroubaceae) as an Economically Prospective Source of Natural Pesticides. Diversity, 14(8), 680. https://doi.org/10.3390/d14080680