Taxonomic Composition of Protist Communities in the Coastal Stratified Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea) Revealed by Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Studied Water Body
2.2. Measurement of Physicochemical Parameters and Sampling
2.3. Treatment of Samples, Microscopy, and Identification of Protists
3. Results
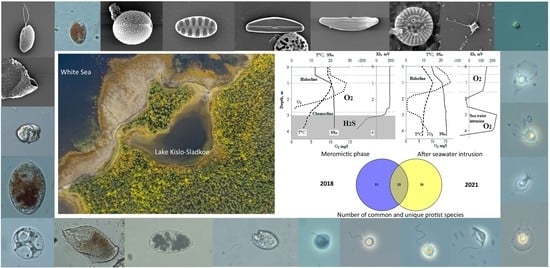
3.1. Hydrological Features of Lake Kislo-Sladkoe, Hydro-Chemical and Hydro-Physical Parameters in the Water Column
3.2. Taxonomic Composition of Protist Communities
3.3. Vertical Distribution of Protists in July 2018
3.4. Vertical Distribution of Protists in September 2021
3.5. Comparison of the Protist Communities in 2018 and 2021
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, K.F.; Likens, G.E. Meromixis and a reconsidered typology of lake circulation patterns. SIL Proc. 1922–2010 1975, 19, 442–458. [Google Scholar] [CrossRef]
- Zadereev, E.S.; Boehrer, B.; Gulati, R.D. Introduction: Meromictic lakes, their terminology and geographic distribution. In Ecology of Meromictic Lakes; Gulati, R.D., Zadereev, E., Degermendzhi, A.G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 228, pp. 1–11. ISBN 978-3-319-49141-7. [Google Scholar]
- Hakala, A. Meromixis as a part of lake evolution-observations and a revised classification of true meromictic lakes in Finland. Boreal Environ. Res. 2004, 9, 37–53. [Google Scholar]
- Krasnova, E.D.; Kharcheva, A.V.; Milyutina, I.A.; Voronov, D.A.; Patsaeva, S.V. Study of microbial communities in redox zone of meromictic lakes isolated from the White Sea using spectral and molecular methods. J. Mar. Biol. Assoc. United Kingd. 2015, 95, 1579–1590. [Google Scholar] [CrossRef]
- Krasnova, E.D. Ecology of meromictic lakes of Russia. 1. Coastal marine waterbodies. Water Resour. 2021, 48, 427–438. [Google Scholar] [CrossRef]
- Fenchel, T.; Kristensen, L.D.; Rasmussen, L. Water column anoxia: Vertical zonation of planktonic protozoa. Mar. Ecol. Prog. Ser. 1990, 62, 1–10. [Google Scholar] [CrossRef]
- Khromechek, E.B.; Barkhatov, Y.V.; Rogozin, D.Y. Community structure and vertical distribution of planktonic ciliates in the saline meromictic lake Shira during breakdown of meromixis. Ecohydrol. Hydrobiol. 2021, 21, 142–152. [Google Scholar] [CrossRef]
- Drljepan, M.; McCarthy, F.M.G.; Hubeny, J.B. Natural and cultural eutrophication of Sluice Pond, Massachusetts, USA, recorded by algal and protozoan microfossils. Holocene 2014, 24, 1731–1742. [Google Scholar] [CrossRef]
- Zadereev, E.S.; Gulati, R.D.; Camacho, A. biological and ecological features, trophic structure and energy flow in meromictic lakes. In Ecology of Meromictic Lakes; Gulati, R.D., Zadereev, E., Degermendzhi, A.G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 61–86. [Google Scholar]
- Fenchel, T.; Bernard, C.; Esteban, G.; Finlay, B.J.; Hansen, P.J.; Iversen, N. Microbial diversity and activity in a Danish Fjord with anoxic deep water. Ophelia 1995, 43, 45–100. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Kosolapov, D.B.; Romanenko, A.V.; Degermendzhy, A.G. Structure of planktonic microbial food web in a brackish stratified siberian lake. Aquat. Ecol. 2002, 36, 179–204. [Google Scholar] [CrossRef]
- Behnke, A.; Bunge, J.; Barger, K.; Breiner, H.-W.; Alla, V.; Stoeck, T. Microeukaryote community patterns along an O2/H2S gradient in a supersulfidic anoxic fjord (Framvaren, Norway). Appl. Environ. Microbiol. 2006, 72, 3626–3636. [Google Scholar] [CrossRef] [Green Version]
- Degermendzhy, A.G.; Zadereev, E.S.; Rogozin, D.Y.; Prokopkin, I.G.; Barkhatov, Y.V.; Tolomeev, A.P.; Khromechek, E.B.; Janse, J.H.; Mooij, W.M.; Gulati, R.D. Vertical stratification of physical, chemical and biological components in two saline Lakes Shira and Shunet (South Siberia, Russia). Aquat. Ecol. 2010, 44, 619–632. [Google Scholar] [CrossRef]
- Khromechek, E.B.; Barkhatov, Y.V.; Rogozin, D.Y. Densities and distribution of flagellates and ciliates in the chemocline of saline, meromictic Lake Shunet (Siberia, Russia). Aquat. Ecol. 2010, 44, 497–511. [Google Scholar] [CrossRef]
- Edgcomb, V.P.; Pachiadaki, M. Ciliates along oxyclines of permanently stratified marine water columns. J. Eukaryot. Microbiol. 2014, 61, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Bykova, S.V. Ecological Specificity and Spatial and Temporal distribution of ciliates from the pelagic plankton of a fresh meromictic waterbody. Inl. Water Biol. 2015, 8, 166–176. [Google Scholar] [CrossRef]
- Umanskaya, M.V.; Bykova, S.V.; Gorbunov, M.Y. Stratification and vertical distribution of ciliates and phototrophic bacteria in boreal ferruginous lake. Water Chem. Ecol. 2018, 117, 74–83. [Google Scholar]
- Adl, S.M.; Simpson, A.G.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Croome, R.L.; Tyler, P.A. Taxonomy and ecology of the phytoplankton of Lake Fidler and Sulphide Pool, meromictic Tasmanian Lakes. Hydrobiologia 1986, 140, 135–141. [Google Scholar] [CrossRef]
- Croome, R.L.; Tyler, P.A. Phyloflagellates and their ecology in Tasmanian polyhumic lakes. Hydrobiologia 1988, 161, 245–253. [Google Scholar] [CrossRef]
- Bowling, L.C.; Tyler, P.A. Lake Chisholm, a polyhumic forest lake in Tasmania. Hydrobiologia 1988, 161, 55–67. [Google Scholar] [CrossRef]
- Burch, M.D. Annual Cycle of phytoplankton in Ace Lake, an ice covered, saline meromictic lake. In Biology of the Vestfold Hills, Antarctica. Developments in Hydrobiology; Ferris, J.M., Burton, H.R., Johnstone, G.W., Bayly, I.A.E., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 34, pp. 59–75. [Google Scholar]
- Rott, E. Some aspects of the seasonal distribution of flagellates in Mountain Lakes. In Flagellates in Freshwater Ecosystems. Developments in Hydrobiology; Jones, R.I., Ilmavirta, V., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 45, pp. 159–170. [Google Scholar]
- Tardio, M.; Tolotti, M.; Novarino, G.; Cantonati, M. Ecological and taxonomic observations on the flagellate algae characterising four years of enclosure experiments in Lake Tovel (Southern Alps). Hydrobiologia 2003, 502, 285–296. [Google Scholar] [CrossRef]
- McCarthy, F.M.G.; Drljepan, M.; Hubeny, J.B.; Krueger, A.M.; Pilkington, P.M.; Riddick, N.L.; MacKinnon, M.D. The influence of dissolved oxygen on dinoflagellate cyst distribution across Sluice Pond, a meromictic lake in NE Massachusetts, USA. Palynology 2017, 41. [Google Scholar] [CrossRef]
- Psennerl, R.; Schlott-Idl, K. Trophic relationships between bacteria and protozoa in the hypolimnion of a meromictic mesostrophic lake. Hydrobiologia 1985, 121, 111–120. [Google Scholar] [CrossRef]
- Carey, P.G.; Sargent, A.J.; Taberner, A.M.; Ramón, G.; Moyà, G. Ecology of cavernicolous ciliates from the anchihaline lagoons of Mallorca. Hydrobiologia 2001, 448, 193–201. [Google Scholar] [CrossRef]
- Stoeck, T.; Bruemmer, F.; Foissner, W. Evidence for local Ciliate endemism in an alpine anoxic lake. Microb. Ecol. 2007, 54, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Laybourn-Parry, J.; Quayle, W.; Henshaw, T. The Biology and evolution of Antarctic saline lakes in relation to salinity and trophy. Polar Biol. 2002, 25, 542–552. [Google Scholar] [CrossRef]
- Saccà, A.; Guglielmo, L.; Bruni, V. Vertical and temporal microbial community patterns in a meromictic coastal lake influenced by the Straits of Messina upwelling system. Hydrobiologia 2008, 600, 89–104. [Google Scholar] [CrossRef]
- Sacca, A.; Borrego, C.M.; Renda, R.; Triado-Margarit, X.; Bruni, V.; Guglielmo, L. Predation impact of ciliated and flagellated protozoa during a summer bloom of brown sulfur bacteria in a meromictic coastal lake. FEMS Microbiol. Ecol. 2009, 70, 42–53. [Google Scholar] [CrossRef]
- Prokopkin, I.G.; Barkhatov, Y.V.; Khromechek, E.B. A one-dimensional model for phytoflagellate distribution in the meromictic lake. Ecol. Model. 2014, 288, 1–8. [Google Scholar] [CrossRef]
- Fenchel, T.; Esteban, D.F.; Finaly, B.J. Local versus global diversity of microorganisms: Cryptic diversity of ciliated protozoa. Oikos 1997, 80, 220–225. [Google Scholar] [CrossRef]
- Vørs, N. Heterotrophic Amoebae, Flagellates and Heliozoa from the Tvärminne Area, Gulf of Finland, in 1988–1990. Ophelia 1992, 36, 1–109. [Google Scholar] [CrossRef]
- Stoeck, T.; Taylor, G.T.; Epstein, S.S. Novel eukaryotes from a permanently anoxic Cariaco Basin (Caribbean Sea). Appl. Environ. Microbiol. 2003, 69, 5656–5663. [Google Scholar] [CrossRef] [Green Version]
- Edgcomb, V.; Orsi, W.; Leslin, C.; Epstein, S.S.; Bunge, J.; Jeon, S.; Yakimov, M.M.; Behnke, A.; Stoeck, T. Protistan community patterns within the brine and halocline of deep hypersaline anoxic basins in the eastern Mediterranean Sea. Extremophiles 2009, 13, 151–167. [Google Scholar] [CrossRef]
- Stock, A.; Jürgens, K.; Bunge, J.; Stoeck, T. Protistan diversity in suboxic and anoxic waters of the Gotland Deep (Baltic Sea) as revealed by 18S rRNA clone libraries. Aquat. Microb. Ecol. 2009, 55, 267–284. [Google Scholar] [CrossRef]
- Behnke, A.; Barger, K.J.; Bunge, J.; Stoeck, T. Spatio-temporal variations in protistan communities alongan O2/H2S gradient in theanoxic Framvaren Fjord (Norway). FEMS Microbiol. Ecol. 2010, 72, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Wylezich, C.; Jürgens, K. Protist diversity in suboxic and sulfidic waters of the Black Sea. Environ. Microbiol. 2011, 13, 2939–2956. [Google Scholar] [CrossRef]
- Orsi, W.; Song, Y.C.; Hallam, S.; Edgcomb, V. Effect of oxygen minimum zone formation on communities of marine protists. ISME J. 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Okamura, T.; Mori, Y.; Nakano, S.I.; Kondo, R. Abundance and bacterivory of heterotrophic nanoflagellates in the meromictic Lake Suigetsu, Japan. Aquat. Microb. Ecol. 2012, 66, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Takishita, K.; Tsuchiya, M.; Kawato, M.; Oguri, K.; Kitazato, H.; Maruyama, T. Genetic diversity of microbial eukaryotes in anoxic sediment of the saline meromictic Lake Namako-Ike (Japan): On the detection of anaerobic or anoxic-tolerant lineages of eukaryotes. Protist 2007, 158, 51–64. [Google Scholar] [CrossRef]
- Edgcomb, V.; Orsi, W.; Jeon, S.; Leslin, C.; Bunge, J.; Taylor, G.T.; Varela, R.; Epstein, S. Protistan microbial observatory in the Cariaco Basin, Caribbean. II. Habitat specialization. ISME J. 2011, 5, 1357–1373. [Google Scholar] [CrossRef] [Green Version]
- Charvet, S.; Vincent, W.F.; Comeau, A.; Lovejoy, C. Pyrosequencing analysis of the protist communities in a High Arctic meromictic lake: DNA preservation and change. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Triadó-Margarit, X.; Casamayor, E.O. High protists diversity in the plankton of sulfurous lakes and lagoons examined by 18S rRNA gene sequence analyses. Environ. Microbiol. Rep. 2015, 7, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, A.; Filker, S.; Breiner, H.-W.; Stoeck, T. Protistan diversity in a permanently stratified meromictic lake (Lake Alatsee, SW Germany). Environ. Microbiol. 2015, 17, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Lepère, C.; Domaizon, I.; Hugoni, M.; Vellet, A.; Debroas, D. Diversity and dynamics of active small microbial eukaryotes in the anoxic zone of a freshwater meromictic lake (Pavin, France). Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, T.; Kondo, R. Protistan Community composition in anoxic sediments from three salinity- disparate Japanese lakes. Estuar. Coast. Shelf Sci. 2019, 224, 34–42. [Google Scholar] [CrossRef]
- Santoferrara, L.; Burki, F.; Filker, S.; Logares, R.; Dunthorn, M.; McManus, G.B. Perspectives from ten years of protist studies by High-throughput metabarcoding. J. Eukaryot. Microbiol. 2020, 67, 612–622. [Google Scholar] [CrossRef]
- Zou, S.; Fu, R.; Deng, H.; Zhang, Q.; Gentekaki, E.; Gong, J. Coupling between ribotypic and phenotypic traits of protists across life cycle stages and temperatures. Microbiol. Spectr. 2021, 9, e01738-21. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Miracle, M.R.; Vicente, E. The Meromictic lake La Cruz (Central Spain). Patterns of stratification. Aquat. Sci. 2001, 63, 406–416. [Google Scholar] [CrossRef]
- Ivanova, D.A.; Krasnova, E.D.; Voronov, D.A.; Radchenko, I.G. Seasonal dynamics of algal flora in the stratified Lake Kislo-Sladkoe partially separated from the White Sea. Oceanology 2022, 62, 207–220. [Google Scholar] [CrossRef]
- Repkina, T.; Shilova, O.; Krasnova, E.; Entin, A.; Grigoriev, V.; Vakhrameyeva, E.; Losyuk, G.; Kublitskiy, Y.; Leontiev, P.; Lugovoy, N.; et al. From the sea strait to the meromictic lake: Evolution and ecosystem of a water body at the Fiard Coast (Lake Kislo-Sladkoe at the Karelian Coast of the Kandalaksha Bay, the White Sea, Russia). Quat. Int. J. 2022; in press. [Google Scholar] [CrossRef]
- Repkina, T.; Krasnova, E.; Leontev, P.; Entin, A.; Alyautdinov, A.; Efimova, L.; Frolova, N.; Lugovoy, N.; Romanenko, F.; Voronov, D. From the strait to the meromictic lake: Water bodies of fjard and skerry coasts, their relief, hydrological features, and ecological communities (on the example of Lake Kislo-Sladkoe, Karelian Coast of the White Sea, Russia). Limnol. Freshw. Biol. 2020, 495–497. [Google Scholar] [CrossRef]
- Krasnova, E.D.; Pantyulin, A.N.; Matorin, D.N.; Todorenko, D.A.; Belevich, T.A.; Milyutina, I.A.; Voronov, D.A. Cryptomonad alga Rhodomonas Sp. (Cryptophyta, Pyrenomonadaceae) bloom in the redox zone of the basins separating from the White Sea. Microbiol. 2014, 83, 270–277. [Google Scholar] [CrossRef]
- Shilova, O.; Leontiev, P.; Vakhrameeva, E.; Losyuk, G.; Grigoriev, V.; Krasnova, E.; Repkina, T.; Kublitskiy, U. From the lagoon to the meromictic lake: A case study of lake-bottom sediments of Lake Kislo-Sladkoe (the Karelian Coast of White Sea, Russia). Limnol. Freshw. Biol. 2020, 490–491. [Google Scholar] [CrossRef]
- Stewart, K.M.; Walker, K.F.; Likens, G.E. Meromictic lakes. In Encyclopedia of Inland Waters; Acad. Press: Oxford, UK, 2009; pp. 589–602. [Google Scholar]
- Losyuk, G.N.; Kokryatskaya, N.M.; Krasnova, E.D. Hydrogen sulfide contamination of coastal lakes at different stages of isolation from the White Sea. Oceanology 2021, 61, 351–361. [Google Scholar] [CrossRef]
- Balonov, M.I. Preparation of Algae for Electron Microscopy. In Methods for the Study of Biocenosis; Nauka: Moscow, Russia, 1975; pp. 87–89. (In Russian) [Google Scholar]
- Preisig, H.R.; Hibberd, D.J. Ultrastructure and taxonomy of Paraphysomonas (Chrysophyceae) and related genera 2. Nord. J. Bot. 1982, 2, 601–638. [Google Scholar] [CrossRef]
- Patterson, D.J.; Fenchel, T. Insights into the evolution of heliozoa (Protozoa, Sarcodina) as provided by ultrastructural studies on a new species of flagellate from the genus Pteridomonas. Biol. J. Linn. Soc. 1985, 24, 381–403. [Google Scholar] [CrossRef]
- Prokina, K.I.; Turbanov, I.S.; Tikhonenkov, D.V.; Mylnikov, A.P. Free-living heterotrophic flagellates from Bays of Sevastopol (the Black Sea Littoral). Protistology 2018, 12, 202–222. [Google Scholar] [CrossRef]
- Mylnikov, A.P.; Tikhonenkov, D.V.; Karpov, S.A.; Wylezich, C. Microscopical Studies on Ministeria vibrans Tong, 1997 (Filasterea) highlight the cytoskeletal structure of the common ancestor of Filasterea, Metazoa and Choanoflagellata. Protist 2019, 170, 385–396. [Google Scholar] [CrossRef]
- Vørs, N. Discocelis saleuta Gen. Nov. et Sp. Nov. (Protista Incertae Sedis)—A new heterotrophic marine flagellate. Eur. J. Protistol. 1988, 23, 297–308. [Google Scholar] [CrossRef]
- Lee, W.J. Small Free-Living Heterotrophic flagellates from marine intertidal sediments of the Sydney Region, Australia. Acta Protozool. 2019, 58, 167–189. [Google Scholar] [CrossRef]
- Jung, M.K.; Yin, T.Y.; Moon, S.J.; Park, J.; Yoon, E.Y. Taxonomy and physiology of Oxyrrhis marina and Oxyrrhis maritima in Korean Waters. Water 2021, 13, 2057. [Google Scholar] [CrossRef]
- Andersen, P. Functional biology of the Choanoflagellate Diaphanoeca grandis Ellis. Mar. Microb. Food Webs 1988, 3, 35–49. [Google Scholar]
- Flavin, M.; Nerad, T.A. Reclinomonas americana N. G., N. Sp., a new freshwater heterotrophic flagellate. J. Eukaryot. Microbiol. 1993, 40, 172–179. [Google Scholar] [CrossRef]
- Tong, S.M. Developayella elegans Nov. Gen., Nov. Spec., a new type of heterotrophic flagellate from marine plankton. Eur. J. Protistol. 1995, 31, 24–31. [Google Scholar] [CrossRef]
- Patterson, D.J.; Simpson, A.G.B. Heterotrophic flagellates from coastal marine and hypersaline sediments in Western Australia. Eur. J. Protistol. 1996, 32, 423–448. [Google Scholar] [CrossRef]
- Tong, S.M.; Nygaard, K.; Bernard, C.; Vørs, N.; Patterson, D.J. Heterotrophic flagellates from the water column in Port Jackson, Sydney, Australia. Eur. J. Protistol. 1998, 34, 162–194. [Google Scholar] [CrossRef]
- Leadbeater, B.S.C. Choanoflagellate lorica construction and assembly: The nudiform condition. I. Savillea Species. Protist 2008, 159, 259–268. [Google Scholar] [CrossRef]
- Lee, W.J.; Patterson, D.J. Heterotrophic flagellates (protista) from marine sediments of Botany Bay, Australia. J. Nat. Hist. 2000, 34, 483–562. [Google Scholar] [CrossRef]
- LeRoi, J.M.; Hallegraeff, G.M. Scale-bearing nanoflagellates from Southern Tasmanian Coastal Waters, Australia. II. Species of Chrysophyceae (Chrysophyta), Prymnesiophyceae (Haptophyta, Excluding Chrysochromulina) and Prasinophyceae (Chlorophyta). Bot. Mar. 2006, 49, 216–235. [Google Scholar] [CrossRef]
- Todorov, M.; Golemansky, V.; Mitchell, E.A.D.; Heger, T.J. Morphology, biometry, and taxonomy of freshwater and marine interstitial Cyphoderia (Cercozoa: Euglyphida). J. Eukaryot. Microbiol. 2009, 56, 279–289. [Google Scholar] [CrossRef]
- Leadbeater, B.S.C.; Cheng, R. Costal strip production and lorica assembly in the large tectiform choanoflagellate Diaphanoeca grandis Ellis. Eur. J. Protistol. 2010, 46, 96–110. [Google Scholar] [CrossRef]
- Scoble, J.M.; Cavalier-Smith, T. Scale Evolution in Paraphysomonadida (Chrysophyceae): Sequence phylogeny and revised taxonomy of Paraphysomonas, new genus Clathromonas, and 25 New Species. Eur. J. Protistol. 2014, 50, 551–592. [Google Scholar] [CrossRef] [Green Version]
- Gómez, F.; Artigas, L.F. High Diversity of dinoflagellates in the intertidal sandy sediments of Wimereux (north-east English Channel, France). J. Mar. Biol. Assoc. United Kingd. 2014, 94, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Zlatogursky, V.V. There and back again: Parallel evolution of cell coverings in centrohelid heliozoans. Protist 2015, 167, 51–66. [Google Scholar] [CrossRef]
- Prokina, K.I.; Mylnikov, A.P.; Zelalem, W. First data on heterotrophic flagellates and heliozoans of Ethiopia. Biol. Bull. 2017, 44, 896–912. [Google Scholar] [CrossRef]
- Prokina, K.I.; Mylnikov, A.P. Heterotrophic flagellates of sphagnum bogs and lakes in Usman Pine Forest, Voronezh Oblast. Inl. Water Biol. 2017, 10, 182–191. [Google Scholar] [CrossRef]
- Ács, É.; Ector, L.; Kiss, K.T.; Cserháti, C.; Morales, E.A.; Levkov, Z. Morphological observations and emended description of Amphora Micrometra from the Bolivian Altiplano, South America. Diatom Res. 2011, 26, 199–212. [Google Scholar] [CrossRef]
- Alves-da-Silva, S.M.; de Mattos Bicudo, C.E. Taxonomic and ultrastructural survey of Trachelomonas (Euglenophyceae) of a shallow subtropical reservoir in the Municipality of Triunfo, Southern Brazil. Rev. Bras. Bot. 2013, 36, 223–246. [Google Scholar] [CrossRef]
- Gogorev, R.M.; Lange, E.K. Centric and araphid diatoms (Bacillariophyta) in water column of the relict Lake Mogilnoye (Kildin Island, Barents Sea). Nov. Sist. Nizshikh Rastenii 2014, 48, 66–80. [Google Scholar] [CrossRef]
- Gogorev, R.M.; Lange, E.K. Naviculoid diatoms (Bacillariophyta) of relict Lake Mogilnoye (Kildin Island, Barents Sea). Nov. Sist. Nizshikh Rastenii 2018, 52, 13–32. [Google Scholar] [CrossRef]
- Gogorev, R.M.; Lange, E.K. Amphoroid and canal-raphid diatoms (Bacillariophyta) of relict Lake Mogilnoye (Kildin Island, Barents Sea). Nov. Sist. Nizshikh Rastenii 2019, 53, 15–38. [Google Scholar] [CrossRef]
- Krammer, K.; Lange, B.H. Bacillariophyceae. 1. Teil: Naviculaceae. Süßwasserflora von Mitteleuropa. Bd. 2/1.; Spektrum Akademischer: Stuttgart, Germany; Jena, Germany, 1986. (In German) [Google Scholar]
- Krammer, K.; Lange, B.H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. Süßwasserflora von Mitteleuropa. Bd. 2/2.; Spektrum Akademischer: Stuttgart, Germany; Jena, Germany, 1988. (In German) [Google Scholar]
- Krammer, K.; Lange, B.H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. Süßwasserflora von Mitteleuropa Bd. 2/3.; Spektrum Akademischer: Stuttgart, Germany; Jena, Germany, 1991. (In German) [Google Scholar]
- Krammer, K.; Lange, B.H. Bacillariophyceae 4. Teil: Achnanthaceae, Kritische Ergänzungen Zu Navicula (Lineolatae) Und Gomphonema Gesamtliteraturverzeichnis Teil 1–4. Süsswasserflora von Mitteleuropa. Bd 2/4.; Spektrum Akademischer: Stuttgart, Germany; Jena, Germany, 1991. (In German) [Google Scholar]
- Kulikovskiy, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification Book of Diatoms from Russia; Filigran: Yaroslavl, Russia, 2016. (In Russian) [Google Scholar]
- Chudaev, D.A.; Gololobova, M.A. Diatoms of Lake Glubokoe (Moscow Region); KMK Scientific Press Ltd.: Moscow, Russia, 2015. (In Russian) [Google Scholar]
- Genkal, S.I.; Kulikovskiy, M.S.; Kuznetsova, I.V. The Recent Freshwater Centric Diatoms of Russia; Filigran: Yaroslavl, Russia, 2020. (In Russian) [Google Scholar]
- Gogorev, R.M. Seasonal changes in phythoplankton from the Chupa Inlet of the White Sea. Nov. Sist. Nizshikh Rastenii 2005, 38, 38–47. (In Russian) [Google Scholar]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/ (accessed on 6 November 2022).
- Patterson, D.J.; Nygaard, K.; Steinberg, G. Heterotrophic flagellates and other protists associated with oceanic detritus throughout the water column in the Mid North Atlantic. J. Mar. Biol. Assoc. United Kingd. 1993, 73, 67–95. [Google Scholar] [CrossRef]
- Tong, S.M. Heterotrophic flagellates and other protists from Southampton Water, U.K. Ophelia 1997, 47, 71–131. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Leander, B.S. Ebriid phylogeny and the expansion of the Cercozoa. Protist 2006, 157, 279–290. [Google Scholar] [CrossRef]
- Krasnova, E.D.; Matorin, D.N.; Belevich, T.A.; Efimova, L.E.; Kharcheva, A.V.; Kokryatskaya, N.M.; Losyuk, G.N.; Todorenko, D.A.; Voronov, D.A.; Patsaeva, S.V. The characteristic pattern of multiple colored layers in coastal stratified lakes in the process of separation from the White Sea. J. Oceanol. Limnol. 2018, 36, 1962–1977. [Google Scholar] [CrossRef]
- Lunina, O.N.; Savvichev, A.S.; Kuznetsov, B.B.; Pimenov, N.V.; Gorlenko, V.M. Anoxygenic phototrophic bacteria of the Kislo-Sladkoe stratified lake (White Sea, Kandalaksha Bay). Mikrobiol. 2013, 82, 815–832. [Google Scholar] [CrossRef]
- Lunina, O.N.; Savvichev, A.S.; Krasnova, E.D.; Kokryatskaya, N.M.; Veslopolova, E.F.; Kuznetsov, B.B.; Gorlenko, V.M. Succession processes in the anoxygenic phototrophic bacterial community in Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea). Microbiol. 2016, 85, 570–582. [Google Scholar] [CrossRef]
- Arnous, M.-B.; Courcol, N.; Carrias, J.-F. The significance of transparent exopolymeric particles in the vertical distribution of bacteria and heterotrophic nanoflagellates in Lake Pavin. Aquat. Sci. 2010, 72, 245–253. [Google Scholar] [CrossRef]
- Tikhonenkov, D.V.; Mazei, Y.A.; Mylnikov, A.P. Species diversity of heterotrophic flagellates in White Sea littoral sites. Eur. J. Protistol. 2006, 42, 191–200. [Google Scholar] [CrossRef]
- Tikhonenkov, D.V.; Mazei, Y.A. Distribution of heterotrophic flagellates at the littoral of estuary of Chernaya River (Kandalaksha Bay, White Sea). Russ. J. Mar. Biol. 2006, 32, 276–283. [Google Scholar] [CrossRef]
- Azovsky, A.; Saburova, M.; Tikhonenkov, D.; Khazanova, K.; Esaulov, A.; Mazei, Y. Composition, diversity and distribution of microbenthos across the intertidal zones of Ryazhkov Island (the White Sea). Eur. J. Protistol. 2013, 49, 500–515. [Google Scholar] [CrossRef]
- Ilyash, L.V.; Radchenko, I.G.; Shevchenko, V.P.; Lisitzin, A.P.; Paka, V.T.; Burenkov, V.I.; Novigatskiy, A.N.; Chul’tsova, A.L.; Pantyulin, A.N. Spatial distribution of phytoplankton in the White Sea in the late summer period with regard to the water structure and dynamics. Oceanology 2011, 51, 993–1003. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, H.; Hu, Z.; Liu, G. Blooms of the woloszynskioid dinoflagellate Tovellia diexiensis sp. nov. (Dinophyceae) in Baishihai Lake at the eastern edge of Tibetan Plateau. Algae 2016, 31, 205–217. [Google Scholar] [CrossRef]
- Fermani, P.; Metz, S.; Balague, V.; Descy, J.P.; Morana, C.; Logares, R.; Massana, R.; Sarmento, H. Microbial eukaryote assemblages and potential novel diversity in four tropical East African Great Lakes. FEMS Microbiol. Ecol. 2021, 97, fiab114. [Google Scholar] [CrossRef]
- Camacho, A.; Miracle, M.R.; Romero-Viana, L.; Picazo, A.; Vicente, E. Lake La Cruz, an iron-rich karstic meromictic lake in Central Spain. In Ecology of Meromictic Lakes; Gulati, R.D., Zadereev, E., Degermendzhi, A.G., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 187–233. [Google Scholar]
- Rengefor, K.; Laybourn-Parry, J.; Logares, R.; Marshall, W.A.; Hansen, G. Marine-derived dinoflagellates in antarctic saline lakes: Community composition and annual dynamics. J. Phycol. 2008, 44, 592–604. [Google Scholar] [CrossRef]
- Hill, D.R.A. Rhodomonas baltica Karsten (Cryptophyceae). Baltic Sea Phytoplankton identification Sheet No. 9. Ann. Bot. Fenn. 1992, 29, 167–168. [Google Scholar]
- Hill, D.R.A.; Wetherbee, R. A reappraisal of the genus Rhodomonas (Cryptophyceae). Phycologia 1989, 28, 143–158. [Google Scholar] [CrossRef]
- Majaneva, M.; Remonen, I.; Rintala, J.M.; Belevich, I.; Kremp, A.; Setälä, O.; Jokitalo, E.; Blomster, J. Rhinomonas nottbecki n. Sp. (Cryptomonadales) and molecular phylogeny of the family Pyrenomonadaceae. J Eukaryot Microbiol 2014, 5, 480–492. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mindolina, Y.V.; Selivanova, E.A.; Ignatenko, M.E.; Krasnova, E.D.; Voronov, D.A.; Plotnikov, A.O. Taxonomic Composition of Protist Communities in the Coastal Stratified Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea) Revealed by Microscopy. Diversity 2023, 15, 44. https://doi.org/10.3390/d15010044
Mindolina YV, Selivanova EA, Ignatenko ME, Krasnova ED, Voronov DA, Plotnikov AO. Taxonomic Composition of Protist Communities in the Coastal Stratified Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea) Revealed by Microscopy. Diversity. 2023; 15(1):44. https://doi.org/10.3390/d15010044
Chicago/Turabian StyleMindolina, Yulia V., Elena A. Selivanova, Marina E. Ignatenko, Elena D. Krasnova, Dmitry A. Voronov, and Andrey O. Plotnikov. 2023. "Taxonomic Composition of Protist Communities in the Coastal Stratified Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea) Revealed by Microscopy" Diversity 15, no. 1: 44. https://doi.org/10.3390/d15010044
APA StyleMindolina, Y. V., Selivanova, E. A., Ignatenko, M. E., Krasnova, E. D., Voronov, D. A., & Plotnikov, A. O. (2023). Taxonomic Composition of Protist Communities in the Coastal Stratified Lake Kislo-Sladkoe (Kandalaksha Bay, White Sea) Revealed by Microscopy. Diversity, 15(1), 44. https://doi.org/10.3390/d15010044