Spider Diversity in the Fragmented Forest-Steppe Landscape of Northeastern Ukraine: Temporal Changes under the Impact of Human Activity
Abstract
:1. Introduction
History of Spider Research in the Study Area
2. Materials and Methods
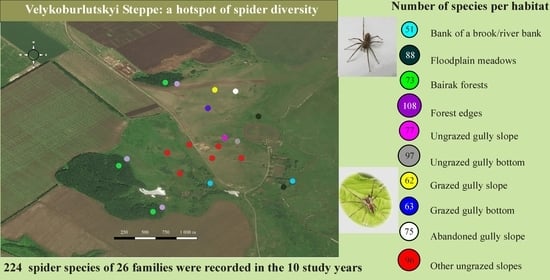
2.1. Study Area
2.2. Sample Plots
- Ungrazed gully bottom (≈137 m a.s.l.); chernozem soil. Abandoned in 1993; grazing pressure before the abandonment was weak (1–1.5 cows/ha). Part of the gully neighboring the sample plot is covered with arboreal vegetation (Pyrus communis, Malus domestica).
- Ungrazed gully slope (≈155 m a.s.l.); strongly washed chernozem or clayey soil. Abandoned in 1991; grazing pressure before the abandonment was weak.
- Grazed gully bottom (≈136 m a.s.l.); chernozem soil. Grazing was intensive (4–5 cows/ha) until abandonment in 2015.
- Grazed gully slope (≈165 m a.s.l.); washed chernozem. Grazing pressure decreased from moderate (2–3 cows/ha) to weak, becoming irregular since 2011. During 2013 and 2014, the grazing area gradually shrank until finally abandoned in 2015.
- Abandoned gully slope (≈160 m a.s.l.), washed chernozem. Grazing pressure decreased from moderate to weak, becoming irregular since 2005. It was abandoned in 2013.
2.3. Spider Collection
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deák, B.; Rádai, Z.; Lukács, K.; Kelemen, A.; Kiss, R.; Bátori, Z.; Kiss, P.J.; Valko, O. Fragmented dry grasslands preserve unique components of plant species and phylogenetic diversity in agricultural landscapes. Biol. Conserv. 2020, 29, 4091–4110. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelgaet, J.-P.; Wingender, W.; Schweiger, O.; Speelmans, M.; Aviron, S.; Augenstein, U.; Billeter, R.; Bailey, D.; Bukacek, R.; et al. Landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Lehikoinen, P.; Tiusanen, M.; Santangeli, A.; Rajasärkkä, A.; Jaatinen, K.; Valkama, J.; Virkkala, R.; Lehikoinen, A. Increasing protected area coverage mitigates climate-driven community changes. Biol. Conserv. 2021, 253, 108892. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.W.; van Mantgem, P.I. The Value of Small Preserves in Chronically Fragmented Landscapes. In Conservation in Highly Fragmented Landscapes; Schwartz, M., Ed.; Chapman & Hall: New York, NY, USA, 1997; Chapter 16; pp. 279–394. [Google Scholar]
- Travassos-de-Britto, B.; da Rocha, P.L.B. Habitat amount, habitat heterogeneity, and their effects on arthropod species diversity. Ecoscience 2013, 20, 207–214. [Google Scholar] [CrossRef]
- Ronkin, V.; Savchenko, G. Flora and vegetation of dry grasslands of Northeastern Ukraine, and problems of diversity conservation. Hacquetia 2016, 15, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Ronkin, V.; Tokarsky, V.; Polchaninova, N.; Atemasov, A.; Koshkina, A.; Savchenko, G. Comparative assessment of ecological plasticity of the steppe marmot between Ukrainian and Kazakhstan populations: Challenges of the man-induced environmental changes. Front. Ecol. Evol. 2020, 8, 219. [Google Scholar] [CrossRef]
- Yang, L.H.; Gratton, C. Insects as drivers of ecosystem processes. Curr. Opin. Insect. Sci. 2014, 2, 26–32. [Google Scholar] [CrossRef]
- Wilson, E.O. The little things that run the world (the importance and conservation of invertebrates). Conserv. Biol. 1987, 1, 344–346. [Google Scholar] [CrossRef]
- Clark, J.A.; May, R.M. Taxonomic bias in conservation research. Science 2002, 297, 191–192. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Burnett, N.J.; Braun, D.C.; Suski, C.D.; Hinch, S.G.; Cooke, S.J.; Kerr, J.T. Taxonomic bias and international biodiversity conservation research. Facets 2016, 1, 105–113. [Google Scholar] [CrossRef]
- Titley, M.A.; Snaddon, J.L.; Turner, E.C. Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS ONE 2017, 12, e0189577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Eliza, M.; Grames, E.M.; Douglas, H.; Boyes, D.H.; Saunders, M.E.; Taylor, N.G. What evidence exists on conservation actions to conserve insects? A protocol for a systematic map of literature reviews. Environ. Evid. 2020, 9, 30. [Google Scholar] [CrossRef]
- Braschler, B.; Baur, B. Diverse effects of a seven-year experimental grassland fragmentation on major invertebrate groups. PLoS ONE 2016, 11, e0149567. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.H. Spiders in Ecological Webs; Cambridge University Press: New York, NY, USA, 1993. [Google Scholar]
- Branco, V.V.; Cardoso, P. An expert-based assessment of global threats and conservation measures for spiders. Glob. Ecol. Conserv. 2020, 24, e01290. [Google Scholar] [CrossRef]
- Michalko, R.; Pekár, S.; Dul’a, M.; Entling, M.H. Global patterns in the biocontrol efficacy of spiders: A meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 1366–1378. [Google Scholar] [CrossRef]
- Nyffeler, M.; Birkhofer, K. An estimated 400–800 million tons of prey are annually killed by the global spider community. Sci. Nat. 2017, 104, 30. [Google Scholar] [CrossRef] [Green Version]
- Argañaraz, C.I.; Rubio, G.D.; Rubio, M.; Castellarini, F. Ground-dwelling spiders in agroecosystems of the Dry Chaco: A rapid assessment of community shifts in response to land use changes. Biodiversity 2020, 21, 125–135. [Google Scholar] [CrossRef]
- Buchholz, S. Ground spider assemblages as indicators for habitat structure in inland sand ecosystems. Biodivers. Conserv. 2010, 19, 2565–2595. [Google Scholar] [CrossRef]
- Hore, U.; Uniyal, V.P. Use of spiders (Araneae) as indicator for monitoring of habitat conditions in Tarai conservation area, India. Indian For. 2008, 134, 1371–1380. [Google Scholar]
- Nadal, M.F. Jumping spiders (Araneae: Salticidae) as indicators of the conservation status of habitats in Eastern Chaco, Argentina. Ecol. Austral 2022, 32, 1120–1132. [Google Scholar] [CrossRef]
- Polchaninova, N.; Krasova, O.; Lysogor, L.; Atemasova, T. Assessment of the conservation value of dry grassland habitats in the Inhulets river basin (Central Ukraine) based on vegetation and spider research. Hacquetia 2021, 20, 225–242. [Google Scholar] [CrossRef]
- Polchaninova, N.; Prokopenko, E. An updated checklist of spiders (Arachnida: Araneae) of Left-Bank Ukraine. Arachnol. Mitt. 2019, 57, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Kirilenko, V.A.; Legotay, M.V. To the study of the Aranei fauna in the eastern forest-steppe of Ukraine. In Fauna i Ekologia Nasekomykh; Permskiy Universitet: Perm, Russia, 1981; pp. 45–54. (In Russian) [Google Scholar]
- Polchaninova, N.Y. A checklist of the spiders (Araneae) of Kharkov Area (Ukraine). Visnyk Kharkivskogo Natsionalnogo Universytetu Ser. Biol. 2009, 856, 121–135, (In Russian, with English Summary). [Google Scholar]
- Polchaninova, N.Y.; Slutsky, A.I. Addition to the annotated checklist of spiders (Araneae) of Kharkov Region (Ukraine). Visnyk Kharkivskogo Natsionalnogo Universytetu Ser. Biol. 2013, 1056, 120–128, (In Russian, with English Summary). [Google Scholar]
- Polchaninova, N.Y.; Prokopenko, E.V. Catalogue of the Spiders (Arachnida, Aranei) of Left-Bank Ukraine; Arthropoda Selecta. Suppl. No 2; KMK Scientific Press Ltd.: Moscow, Russia, 2013. [Google Scholar]
- Polchaninova, N.Y.; Prokopenko, E.V. Catalogue of the Spiders (Arachnida, Aranei) of Left-Bank Ukraine. Addendum 1. 2013–2016; Arthropoda Selecta. Suppl. No 4; Mikhailov, K.G., Ed.; KMK Scientific Press Ltd.: Moscow, Russia, 2017. [Google Scholar]
- Polchaninova, N.; Savchenko, G.; Drogvalenko, A.; Ronkin, V.; Shabanov, D. The impact of cattle grazing on cursorial spiders (Aranei) and true bugs (Heteroptera) in steppe gullies of northeastern Ukraine. Agric. Ecosyst. Environ. 2016, 234, 65–71. [Google Scholar] [CrossRef]
- Polchaninova, N.; Savchenko, G.; Ronkin, V.; Drogvalenko, A.; Putchkov, A. Summer fire in steppe habitats: A long-term effect on vegetation and autumnal assemblages of cursorial arthropods. Hacquetia 2019, 18, 213–231. [Google Scholar] [CrossRef] [Green Version]
- Tokarsky, V. (Ed.) Red Data Book of Kharkiv Region. Animals; V.N. Karazin Kharkiv National University: Kharkiv, Ukraine, 2013. (In Ukrainian) [Google Scholar]
- Polchaninova, N.Y. Rare spider species (Araneae) of protected steppe areas of the Kharkiv Region (Ukraine). J. V.N. Karazin Kharkiv Nat. Univ. Ser. Biol. 2019, 32, 99–106. [Google Scholar]
- Polchaninova, N.; Gnelitsa, V.; Terekhova, V.; Iosypchuk, A. New and rare spider species (Arachnida: Araneae) from Ukraine. Zoodiversity 2021, 55, 95–112. [Google Scholar] [CrossRef]
- Milano, F.; Blik, T.; Cardoso, P.; Chatzaki, M.; Fukushima, K.S.; Gajdoš, P.; Gibbons, A.T.; Henriques, S.; Macías-Hernández, N.; Mammola, S.; et al. Spider conservation in Europe: A review. Biol. Conserv. 2021, 256, 109020. [Google Scholar] [CrossRef]
- Polchaninova, N. Spiders (Arachnida: Araneae) in dry grasslands of south Ukraine: A case study of Yelanetskyi Steppe Natural Reserve. Arachnol. Mitteil. 2021, 61, 27–35. [Google Scholar] [CrossRef]
- Savchenko, G.; Ronkin, V. Grazing, abandonment and frequent mowing influence the persistence of the steppe marmot, Marmota bobak. Hacquetia 2018, 17, 25–34. [Google Scholar] [CrossRef] [Green Version]
- WSC. World Spider Catalog Version 23.1. Available online: http://www.wsc.nmbe.ch/ (accessed on 10 January 2023).
- Mikhailov, K.G. Advances in the study of the spider fauna (Aranei) of Russia and adjacent regions: A 2020 update. Invert. Zool. 2022, 19, 295–304. [Google Scholar] [CrossRef]
- Polchaninova, N.Y.; Gnelitsa, V.A.; Evtushenko, K.V.; Singaevsky, E.N. An annotated checklist of spiders (Arachnida: Aranei) of the National Nature Park ‘Buzkyi Hard’ (Mykolaiv Area, Ukraine). Arthrop. Sel. 2017, 26, 253–272. [Google Scholar] [CrossRef]
- Ponomarev, A.V. Spiders (Arachnida: Aranei) of steppe and meadow-steppe habitats of gully and ravine ecosystems of the valley of the Don River lower reaches. Proc. Rus. Ent. Soc. 2017, 88, 118–131, (In Russian with English Summary). [Google Scholar]
- Revised Annex I of Resolution 4 (1996) of the Bern Convention on Endangered Natural Habitats Types Using the EUNIS Habitat Classification (Adopted by the Standing Committee on 6 December 2019). Available online: https://rm.coe.int/16807469e7 (accessed on 6 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://folk.uio.no/ohammer/past (accessed on 10 February 2023).
- Mallis, R.E.; Hurd, L.E. Diversity among ground-dwelling spider assemblages: Habitat generalists and specialists. J. Arachnol. 2005, 33, 101–109. [Google Scholar] [CrossRef]
- Jansen, R.; Makaka, L.; Little, I.T.; Dippenaar-Schoema, A. Response of ground-dwelling spider assemblages (Arachnida, Araneae) to Montane Grassland management practices in South Africa. Insect Conserv. Divers. 2013, 6, 572–589. [Google Scholar] [CrossRef] [Green Version]
- Duffey, E.; Fees, A. A comparative ecological study of the spider (Araneae) faunas of East Anglian fens, England: Regional differences and conservation. Bull. Brit. Arachnol. Soc. 2009, 14, 317–333. [Google Scholar] [CrossRef]
- Tischler, W. Grundzüge der Terrestrischen Tierökologie; Vieweg: Braunschweig, Germany, 1949. [Google Scholar]
- Prokopenko, E.V.; Polchaninova, N.Y. The results of studies of the spider fauna (Aranei) in the Nature Reserve ‘Kamiani Mohyly’. In Pryrodna ta istoryko-kulturna spadshchyna rayonu zapovidnyka “Kamyani Mohyly”; Sirenko, V., Kolomiichuk, V., Eds.; Series «Conservation Biology in Ukraine», Issue 4; Schmalhausen Institute of Zoology NAS of Ukraine: Kyiv, Ukraine, 2017; pp. 266–279, (In Russian, with English Summary). [Google Scholar]
- Polchaninova, N. Spiders (Aranei) of the ‘Privolzhskaya Lesostep’ Nature Reserve (Penza Area, Russia): Sector ‘Kuncherovskaya Lesostep’. Arthrop. Sel. 2020, 29, 371–386. [Google Scholar] [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Samways, M.J. Insect Conservation: A Synthetic Management Approach. Annu. Rev. Entomol. 2007, 52, 465–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coddington, J.A.; Agnarsson, I.; Miller, J.A.; Kuntner, M.; Hormiga, G. Undersampling bias: The null hypothesis for singleton species in tropical arthropod surveys. J. Anim. Ecol. 2009, 78, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.; Silva, I.; De Oliveira, N.G.; Serrano, A.R.M. Indicator taxa of spider (Araneae) diversity and their efficiency in conservation. Biol. Conserv. 2004, 120, 517–524. [Google Scholar] [CrossRef]
- Looney, C.; Caldwell, B.T.; Eigenbrode, S.D. When the prairie varies: The importance of site characteristics for strategising insect conservation. Insect Conserv. Div. 2009, 2, 243–250. [Google Scholar] [CrossRef]
- Nentwig, W.; Blick, T.; Bosmans, R.; Gloor, D.; Hänggi, A.; Kropf, C. Spiders of Europe. Version 1. 2023. Available online: https://www.araneae.nmbe.ch (accessed on 15 January 2023).
- Řezáč, M.; Heneberg, P. Grazing as a conservation management approach leads to a reduction in spider species richness and abundance in acidophilous steppic grasslands on andesite bedrock. J. Insect Conserv. 2019, 23, 777–783. [Google Scholar] [CrossRef]
- Pétillon, J.; François, A.; Lafage, D. Short–term effects of horse grazing on spider assemblages of a dry meadow (Western France). Anim. Biodivers. Conserv. 2018, 41, 19–32. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Samu, F.; Szűts, T.; Erdős, S. Are spiders reacting to local or landscape scale effects in Hungarian pastures? Biol. Conserv. 2008, 141, 2062–2070. [Google Scholar] [CrossRef]
- Dennis, P.; Skartveit, J.; Kunaver, A.; McCracken, D.I. The response of spider (Araneae) assemblages to structural heterogeneity and prey abundance in sub-montane vegetation modified by conservation grazing. Glob. Ecol. Conserv. 2015, 3, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Torma, A.; Révész, K.; Gall’e-Szpisjak, N.; Šeat, J.; Szél, G.; Kutasi, C.; Malenovský, I.; Batáry, P.; Gallé, R. Differences in arthropod communities between grazed areas and grazing exclosures depend on arthropod groups and vegetation types. Agric. Ecosyst. Environ. 2023, 341, 108222. [Google Scholar] [CrossRef]





| Number | Abbreviation | Description | Geographic Coordinates | Vegetation (Community of) | Study Period |
|---|---|---|---|---|---|
| DRY GRASSLANDS | |||||
| 1 | UgB | Ungrazed gully bottom; grazing ceased in 1992. Aspect 330°, inclination 3–4° | 49.92499° N; 37.30972° E | Festuca rupicola, Fragaria viridis, Chamaecytisus ruthenicus | April–October 2012–2019 |
| 2 | UgS | Ungrazed gully slope; grazing ceased in 1990. Aspect 170°, inclination 10–12° | 49.92531° N; 37.30829° E | Galatella villosa, Festuca valesiaca aggr. | April–October 2012–2019 |
| 3 | GB | Grazed gully bottom; grazing ceased in 2014. Aspect 90°, inclination 2–3°. | 49.92850° N; 37.30648° E | Festuca valesiaca aggr., Poa angustifolia, Elytrigia repens | April–October 2012–2019 |
| 4 | GS | Grazed gully slope; grazing ceased in 2012–2014. Aspect 200°, inclination 17° | 49.93078° N; 37.30734° E | Bromopsis inermis, Festuca valesiaca aggr., Poa angustifolia, Medicago romanica | April–October 2012–2016 |
| 5 | AS | Abandoned gully slope; grazing was ceased in 2012. Aspect 120°, inclination 12° | 49.93040° N; 37.30956° E | Stipa capillata, Festuca valesiaca aggr., Pilosella officinarum, Bromopsis inermis | April–October 2013–2016, and 2018–2019 |
| 6 | OUgS | Ungrazed gully slope; grazing was ceased in 1990. Aspect 10°, inclination 12° | 49.92434° N; 37.30761° E | Festuca valesiaca aggr., Festuca rupicola | April–July, September–October 2017, 2018 |
| 7 | OUgS | Ungrazed gully slope; grazing ceased in 2003. Aspect 250°, inclination 8–10° | 49.92455° N; 37.30445° E | Stipa pennata | April–July 2016 |
| 8 | OUgS | Ungrazed gully slope; grazing ceased in 2001. Aspect 10°, inclination 12–15° | 49.92365° N; 37.30208° E | Festuca valesiaca aggr., Fragaria viridis | April–June 2017 |
| 9 | OUgS | Ungrazed gully slope; grazing was ceased in 2003. Aspect 90°, inclination 3–4° | 49.92137° N; 37.30346° E | Festuca rupicola, Poa angustifolia, Bromopsis inermis, Elytrigia repens, Agrimonia eupatoria | April–July 2014 |
| 10 | OUgS | Ungrazed gully slope; grazing was ceased in 2000. Aspect 210°, inclination 3–4° | 49.92307° N; 37.30693° E | Festuca valesiaca aggr., Artemisia marschalliana | June–July 2003, May–July, and October 2008 |
| 11 | OUgS | Ungrazed gully slope; grazing was ceased in 2000. Aspect 90°, inclination 2–3° | 49.92307° N; 37.31009° E | Festuca valesiaca aggr., Calamagrostis epigeios, Carex praecox | May–October 2012 |
| FORESTS | |||||
| 12 | FE | Forest edge | 49.92282° N; 37.29781° E | Prunus spinosa | June–July 2003, May–July, and October 2008 |
| 13 | BF | Forest under the canopy. Lower third of a slope with dense grass cover | 49.92223° N; 37.29713° E | Quercus robur, Acer platanoides, Acer campestre, Ulmus glabra, Corylus avellana, Aegopodium podagraria, Carex pilosa | June–July 2003, May–July, and October 2008 |
| 14 | FE | Forest edge | 49.91731° N; 37.30168° E | Prunus spinosa, Ulmus sp., Swida sanguinea, Acer platanoides, Cerasus avium, Pyrus communis | April–July 2018 |
| 15 | BF | Forest under the canopy. Lower third of a slope with sparse grass cover | 49.91704° N; 37.30116° E | Quercus robur, Acer platanoides, Acer campestre, Cerasus avium, Corylus avellana | April–July 2018 |
| 16 | FE | Forest edge | 49.93089° N; 37.30295° E | Prunus spinosa | April–July 2018, 2019 |
| 17 | BF | Forest under the canopy. Lower third of a slope with sparse grass cover | 49.93146° N; 37.30181° E | Quercus robur, Acer platanoides, Acer campestre, Acer tataricum, Tilia cordata, Euonymus verrucosa, E. europaea, Aegopodium podagraria | April–July 2018, 2019 |
| MEADOWS | |||||
| 18 | FM | Floodplain meadow | 49.92765° N; 37.31251° E | Festuca pratensis, Poa angustifolia, Carex cf. riparia | May–July 2014, April–May 2019 |
| 19 | WB | Bank of a brook | 49.92390° N; 37.30610° E | Phragmites australis, Calamagrostis epigeios | May–July, and October 2008 |
| 20 | FM | Floodplain meadow | 49.92030° N; 37.31527° E | Festuca rupicola | May–July, and October 2008 |
| 21 | WB | Riverbank | 49.92027° N; 37.31551° E | Phragmites australis, Carex cf. riparia | June–July 2003, May–July, and October 2008 |
| OTHER HABITATS | |||||
| 22 | Sn | Dwellings and outbuildings | 49.92474° N; 37.31303° E | May–July, and September–October 2014, 2018, and 2019 | |
| Families | Dry Grasslands | Forests | Wetlands | Buildings | Total | |
|---|---|---|---|---|---|---|
| Slopes | Bottoms | |||||
| of the Gullies | ||||||
| Agelenidae | 1(0.8) | 1(0.9) | 2(1.4) | . | 2 | 3(1.3) |
| Araneidae | 7(5.3) | 9(8.3) | 12(8.7) | 11(10.6) | 4 | 16(7.1) |
| Atypidae | 1(0.8) | . | . | . | . | 1(0.4) |
| Cheiracanthiidae | 2(1.5) | 3(2.8) | 5(3.6) | 3(2.9) | . | 5(2.2) |
| Clubionidae | 1(0.8) | 1(0.9) | 2(1.4) | 5(4.8) | . | 6(2.7) |
| Dictynidae | 4/3.1 | 2(1.8) | 2(1.4) | 3(2.9) | . | 5(2.2) |
| Dysderidae | . | . | . | . | 1 | 1(0.4) |
| Eresidae | 1(0.8) | 1(0.9) | . | . | . | 1(0.4) |
| Gnaphosidae | 28(21.4) | 22(20.2) | 19(13.8) | 13(2.5) | . | 33(14.7) |
| Hahniidae | 1(0.8) | 1(0.9) | 1(0.7) | . | . | 2(0.9) |
| Linyphiidae | 17(13.0) | 14(12.8) | 21(15.2) | 15(14.4) | . | 34(15.2) |
| Liocranidae | 3(2.3) | 2(1.8) | 4(2.9) | 2(1.9) | . | 5(2.2) |
| Lycosidae | 13(9.9) | 15(13.8) | 19(13.8) | 20(19.2) | . | 27(12.1) |
| Mimetidae | . | 1(0.9) | 1(0.7) | . | . | 1(0.4) |
| Miturgidae | 1(0.8) | 2(1.8) | 3(2.2) | 2(1.9) | . | 3(1.3) |
| Philidromidae | 6(4.6) | 5(4.6) | 5(3.6) | 2(1.9) | 1 | 9(4.0) |
| Pholcidae | . | . | . | . | 1 | 1(0.4) |
| Prurolithidae | 2(1.5) | 2(1.8) | 1(0.7) | 1(1.0) | . | 2(0.9) |
| Pisauridae | 1(0.8) | 1(0.9) | 1(0.7) | 1(1.0) | . | 1(0.4) |
| Salticidae | 16(12.2) | 9(8.3) | 11(8.0) | 8(7.7) | . | 21(9.4) |
| Sparassidae | 1(0.8) | 1(0.9) | 1(0.7) | 1(1.0) | . | 1(0.4) |
| Tetragnathidae | . | 1(0.9) | 3(2.2) | 5(4.8) | 5(2.2) | |
| Theridiidae | 14(10.7) | 7(6.4) | 13(9.4) | 3(2.9) | 3 | 21(9.4) |
| Thomisidae | 9(6.9) | 9(8.3) | 11(8.0) | 9(8.7) | . | 17(7.6) |
| Titanoecidae | 1(0.8) | . | 1(0.7) | . | . | 2(0.9) |
| Uloboridae | 1(0.8) | . | . | . | . | 1(0.4) |
| Total | 131(100) | 109(100) | 138(100) | 104(100) | 12 | 224(100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polchaninova, N.; Savchenko, G.; Ronkin, V.; Shabanov, D. Spider Diversity in the Fragmented Forest-Steppe Landscape of Northeastern Ukraine: Temporal Changes under the Impact of Human Activity. Diversity 2023, 15, 351. https://doi.org/10.3390/d15030351
Polchaninova N, Savchenko G, Ronkin V, Shabanov D. Spider Diversity in the Fragmented Forest-Steppe Landscape of Northeastern Ukraine: Temporal Changes under the Impact of Human Activity. Diversity. 2023; 15(3):351. https://doi.org/10.3390/d15030351
Chicago/Turabian StylePolchaninova, Nina, Galina Savchenko, Vladimir Ronkin, and Dmytro Shabanov. 2023. "Spider Diversity in the Fragmented Forest-Steppe Landscape of Northeastern Ukraine: Temporal Changes under the Impact of Human Activity" Diversity 15, no. 3: 351. https://doi.org/10.3390/d15030351
APA StylePolchaninova, N., Savchenko, G., Ronkin, V., & Shabanov, D. (2023). Spider Diversity in the Fragmented Forest-Steppe Landscape of Northeastern Ukraine: Temporal Changes under the Impact of Human Activity. Diversity, 15(3), 351. https://doi.org/10.3390/d15030351