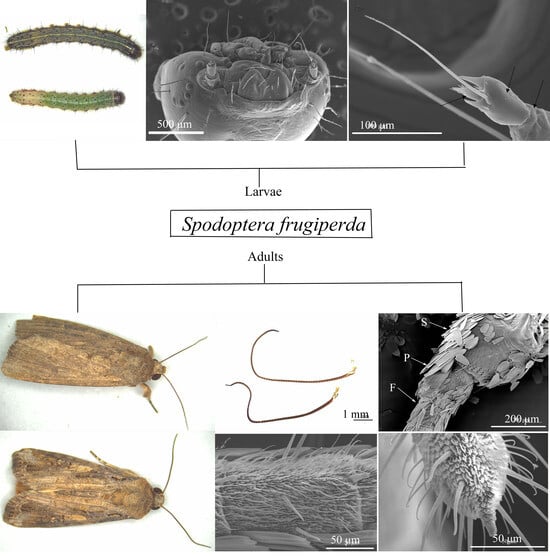
Morphology and Distribution of Antennal Sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae and Adults
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Morphological Characteristics of Antennae in Spodoptera frugiperda Larvae
3.2. The Antennal Sensilla Types and Distribution of Spodoptera frugiperda Larvae
3.2.1. Sensilla Trichodea (ST)
3.2.2. Sensilla Basiconica (SB)
3.2.3. Sensilla Chaetica (SCh)
3.2.4. Sensilla Styloconicum (SSt)
3.2.5. Sensilla Campaniform (SCam)
3.3. General Description of Antennae of FAW Adults
3.4. The Antennal Sensilla Type and Distribution of Spodoptera frugiperda Adults
3.4.1. Böhm’s Bristles (BB)
3.4.2. Sensilla Trichodea (ST)
3.4.3. Sensilla Basiconica (SB)
3.4.4. Sensilla Chaetica (SCh)
3.4.5. Sensilla Coeloconica (SCo)
3.4.6. Sensilla Styloconicum (SSt)
3.4.7. Sensilla Cavity (SCa)
3.4.8. Sensilla Squamous (SSq)
3.4.9. Sensilla Auricillica (SA)
3.4.10. Sensilla Ligulate (SL)
3.4.11. Sensilla Uniporous Peg (SU)
3.4.12. Sensilla Placodea (SPl)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Murúa, M.G.; Nagoshi, R.N.; Dos Santos, D.A.; Hay-Roe, M.M.; Meagher, R.L.; Vilardi, J.C. Demonstration using field collections that argentina fall armyworm populations exhibit strain-specific host plant preferences. J. Econ. Entomol. 2015, 108, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Jiang, C.X.; Guo, X.; Chen, D.D.; You, C.; Zhang, Y.; Wang, M.T.; Li, Q. Potential distribution of Spodoptera frugiperda (J. E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Conserv. 2020, 21, e00865. [Google Scholar] [CrossRef]
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Hardke, J.T.; Jackson, R.E.; Leonard, B.R.; Temple, J.H. Fall armyworm (Lepidoptera: Noctuidae) development, survivorship, and damage on cotton plants expressing insecticidal plant-incorporated protectants. J. Econ. Entomol. 2015, 108, 1086–1093. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Liang, P.; Gu, S.; Zhang, L.; Gao, X. Research status and prospects of Spodoptera frugiperda (Lepidoptera: Noctuidae) in China. Acta Entomol. Sin. 2020, 63, 624–638. [Google Scholar] [CrossRef]
- Sisay, B.; Sevgan, S.; Weldon, C.W.; Krüger, K.; Torto, B.; Tamiru, A. Responses of the fall armyworm (Spodoptera frugiperda) to different host plants: Implications for its management strategy. Pest Manag. Sci. 2023, 79, 845–856. [Google Scholar] [CrossRef]
- Kenis, M. Prospects for classical biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. J. Econ. Entomol. 2023, 116, 331–341. [Google Scholar] [CrossRef]
- Cardoso, V.; Linardi, P.M. Scanning electron microscopy studies of sensilla and other structures of the head of Polygenis (Polygenis) tripus (Siphonapera: Rhopalopsyllidae). Micron 2006, 37, 557–565. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. The antennal sensilla of the adult of Libellula depressa (Odonata: Libellulidae). Arthropod Struct. Dev. 2008, 37, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Diakite, M.M.; Ali, S.; Wang, M.Q. Morphology and ultrastructure of the antennal sensilla of Sitophilus granarius (Coleoptera: Curculionidae). Bull. Entomol. Res. 2016, 106, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.S.; Dou, F.G.; Yang, Y.B.; Wickham, J.D.; Tang, R.; Zhang, Y.J.; Huang, Z.Y.; Zheng, X.L.; Wang, X.Y.; Lu, W. First description and comparison of the morphological and ultramicro characteristics of the antennal sensilla of two fir longhorn beetles. PLoS ONE 2020, 15, e0241115. [Google Scholar] [CrossRef]
- Ribeiro Júnior, C.; Serrão, J.E. Antennal sensilla in Vespidae: A comparison between a diurnal and a nocturnal polistinae wasp. Microsc. Microanal. 2022, 28, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Wu, W.J.; Liang, G.W.; Fu, Y.G. Nymphal antennae and antennal sensilla in Aleurodicus dispersus (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 2014, 104, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, S.; Chakraborty, R. Surface ultrastructure of larval mouthpart sensilla of the muga silkmoth, Antheraea assamensis, an endemic species of North-East India. Microsc. Res. Tech. 2011, 74, 292–300. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Radek, R.; Adler, C. Structure and distribution of antennal sensilla in the Indianmeal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Wee, S.L.; Oh, H.W.; Park, K.C. Antennal sensillum morphology and electrophysiological responses of olfactory receptor neurons in trichoid sensilla of the diamondback moth (Lepidoptera: Plutellidae). Fla. Entomol. 2016, 99, 146–158. [Google Scholar] [CrossRef]
- Ma, L.Y.; Hu, K.; Li, P.D.; Liu, J.Q.; Yuan, X.Q. Ultrastructure of the proboscis sensilla of ten species of butterflies (Insecta: Lepidoptera). PLoS ONE 2019, 14, e0214658. [Google Scholar] [CrossRef]
- Rani, A.T.; Shashank, P.R.; Meshram, N.M.; Sagar, D.; Srivastava, C.; Pandey, K.K.; Singh, J. Morphological characterization of antennal sensilla of Earias vittella (Fabricius) (Lepidoptera: Nolidae). Micron 2021, 140, 102957. [Google Scholar] [CrossRef]
- Malo, E.A.; Castrejongomez, V.R.; Cruzlopez, L.; Rojas, J.C. Antennal sensilla and electrophysiological response of male and female Spodoptera frugiperda (Lepidoptera: Noctuidae) to conspecific sex pheromone and plant pdors. Ann. Entomol. Soc. Am. 2004, 97, 1273–1284. [Google Scholar] [CrossRef]
- Tian, C.H.; Huang, J.R.; Wang, Y.N.; Zhang, S.G.; Li, G.P. Ultrastructure and morphology of antennal sensilla of the adult Spodoptera frugiperda (J. E. Smith). Plant Prot. 2021, 47, 216–221. [Google Scholar] [CrossRef]
- Gargi, C.; Kennedy, J.S.; Jayabal, T.D. Morphometrics and distribution of antennal sensillae of both sexes of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). J. Appl. Nat. Sci. 2022, 14, 41–48. [Google Scholar] [CrossRef]
- Schneider, D. Insect Antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Shields, V.D. Sensilla of immature insects. Annu. Rev. Entomol. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- Ansebo, L.; Ignell, R.; Lofqvist, J.; Hansson, B.S. Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2005, 51, 1066–1074. [Google Scholar] [CrossRef]
- Ronderos, D.; Smith, D. Diverse signaling mechanisms mediate volatile odorant detection in Drosophila. Fly 2009, 3, 290–297. [Google Scholar] [CrossRef]
- Xu, L.L.; Pei, J.H.; Wang, T.; Ren, L.L.; Zong, S.X. The larval sensilla on the antennae and mouthparts of five species of Cossidae (Lepidoptera). Can. J. Zool. 2017, 95, 611–622. [Google Scholar] [CrossRef]
- Zhang, F.M.; Jin, Y.L.; Zhang, L.L.; Yin, J.; Chen, J.H.; Zhao, Q.; Pan, P.L. Ultrastructure of the sensilla on adult antenna and larval head of Ectropis grisescens (Lepidoptera: Geometridae). Acta Entomol. Sin. 2019, 62, 743–755. [Google Scholar] [CrossRef]
- Qin, D.Q.; Zhang, P.W.; Zhou, Y.; Liu, B.J.; Xiao, C.X.; Chen, W.B.; Zhang, Z.X. Antifeeding effects of azadirachtin on the fifth instar Spodoptera litura larvae and the analysis of azadirachtin on target sensilla around mouthparts. Arch. Insect Biochem. Physiol. 2020, 103, e21646. [Google Scholar] [CrossRef]
- Ahmed, T.; Zhang, T.T.; Wang, Z.Y.; He, K.L.; Bai, S.X. Morphology and ultrastructure of antennal sensilla of Macrocentrus cingulum Brischke (Hymenoptera: Braconidae) and their probable functions. Micron 2013, 50, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.J. Electrophysiological responses to sucrose from a gustatory sensillum on the larval maxillary palp of the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). J. Insect Physiol. 2003, 49, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; Dey, S.; Kalita, J. Fine structural studies on major larval mouth part sensilla of Antheraea assamensis, an endemic silk moth species of North East India in regard to sensory physiology. J. Appl. Fundam. Sci. 2016, 2, 6–16. [Google Scholar]
- Dey, S.; Choudhury, S. Physiological significance of gravity receptors on larval cephalic cuticle in the silk moth, Antheraea proylei Jolly. Microsc. Res. Tech. 2018, 81, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Filippis, T.D.; Leite, A.C.R. Scanning electron microscopy studies on the first-instar larva of Dermatobia hominis. Med. Vet. Entomol. 1997, 11, 165–171. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. Comparative studies of ovipositional preference, larval feeding selectivity, and nutritional indices of Spodoptera frugiperda (Lepidoptera: Noctuidae) on 6 crops. J. Econ. Entomol. 2023, 116, 790–797. [Google Scholar] [CrossRef]
- Isidoro, N.; Bartlet, E.; Ziesmann, J.; Williams, I.H. Antennal contact chemosensilla in Psylliodes chrysocephala responding to cruciferous allelochemicals. Physiol. Entomol. 1998, 23, 131–138. [Google Scholar] [CrossRef]
- Ruschioni, S.; Riolo, P.; Verdolini, E.; Peri, E.; Guarino, S.; Colazza, S.; Romani, R.; Isidoro, N. Fine structure of antennal sensilla of Paysandisia archon and electrophysiological responses to volatile compounds associated with host palms. PLoS ONE 2015, 10, e0124607. [Google Scholar] [CrossRef]
- Binyameen, M.; Anderson, P.; Ignell, R.; Seada, M.A.; Hansson, B.S.; Schlyter, F. Spatial organization of antennal olfactory sensory neurons in the female Spodoptera littoralis moth: Differences in sensitivity and temporal characteristics. Chem. Senses 2012, 37, 613–629. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, K.C.; Oh, H.-W.; Park, C.G. Morphology and distribution of antennal sensilla of two tortricid moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 2016, 79, 1069–1081. [Google Scholar] [CrossRef]
- Anderson, P.; Hallberg, E.; Subchev, M. Morphology of antennal sensilla auricillica and their detection of plant volatiles in the Herald moth, Scoliopteryx libatrix L. (Lepidoptera: Noctuidae). Arthropod Struct. Dev. 2000, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, S.C.; Liu, D. Ultrastructural observations on antennal sensilla of Coleophora obducta (Meyrick) (Lepidoptera: Coleophoridae). Micron 2009, 40, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Nacro, S.; Nénon, J.P. Comparative study of the morphology of the ovipositor of Platygaster diplosisae (Hymenoptera: Platygasteridae) and Aprostocetus procerae (Hymenoptera: Eulophidae) two parasitoids associated with the African rice gall midge, Orseolia oryzivora (Diptera: Cecidomyiidae). Psyche A J. Entomol. 2009, 2009, 675242. [Google Scholar] [CrossRef]
- Dong, Z.S.; Yang, Y.B.; Dou, F.G.; Zhang, Y.J.; Huang, H.X.; Zheng, X.L.; Wang, X.Y.; Lu, W. Observations on the ultrastructure of antennal sensilla of adult Glenea cantor (Cerambycidae: Lamiinae). J. Insect Sci. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Xu, L.L.; Zong, S.X.; Luo, Y.Q. Sensilla on the antennae and ovipositor of the sea buckthorn Carpenter moth, Holcocerus hippophaecolus Hua et al. (Lepidoptera: Cossidae). Neotrop. Entomol. 2015, 44, 68–76. [Google Scholar] [CrossRef]
- Jiang, X.J.; Ning, C.; Guo, H.; Jia, Y.Y.; Huang, L.Q.; Qu, M.J.; Wang, C.Z. A gustatory receptor tuned to D-fructose in antennal sensilla chaetica of Helicoverpa armigera. Insect Biochem. 2015, 60, 39–46. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhang, S.; Lv, L.; Wang, M.Q. Insight Into the ultrastructure of antennal sensilla of Mythimna separata (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 124. [Google Scholar] [CrossRef]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo- and hygroreceptors. Annu. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Castrejón-Gómez, V.R.; Valdez-Carrasco, J.; Cibrian-Tovar, J.; Camino-Lavin, M.; Rodolfo Osorio, O. Morphology and distribution of the sense organs on the antennae of Copitarsia consueta (Lepidoptera: Noctuidae). Fla. Entomol. 1999, 82, 546–555. [Google Scholar] [CrossRef]
- Seada, M.A. Antennal morphology and sensillum distribution of female cotton leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). JOBAZ. 2015, 68, 10–18. [Google Scholar] [CrossRef]
- Bai, J.C.; Chen, K.W.; Chen, L.; Liang, G.W.; Zeng, L. Antennal sensilla of Diachasmimorpha longcicaudata (Ashmead) observed with scanning electron microscopy. J. Environ. Entomol. 2012, 34, 339–344. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.Q.; Zhang, G. Ultrastructural observations on antennal sensilla of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2011, 74, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chang, M.M.; Lu, Y.; Lei, C.L.; Yang, F.L. Ultrastructure of sensilla of antennae and ovipositor of Sitotroga cerealella (Lepidoptera: Gelechiidae), and location of female sex pheromone gland. Sci. Rep. 2017, 7, 40637. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.R.; Singh, K.; Singh, R.N. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Res. 1995, 282, 237–249. [Google Scholar] [CrossRef]





| Type | Length (µm) | Basal Diameter (µm) | Basal Column Height (µm) | Peg Height (µm) | Top | Socket | Distribution | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Third-Instar | Fifth-Instar | Third-Instar | Fifth-Instar | Third-Instar | Fifth-Instar | Third-Instar | Fifth-Instar | ||||
| Scape | 15.4 ± 0.6 | 41.6 ± 2.0 * | 41.7 ± 1.6 | 100.4 ± 1.6 * | - | - | - | - | - | - | - |
| Pedicel | 47.7 ± 2.1 | 139.0 ± 2.6 * | 40.5 ± 2.4 | 83.7 ± 1.8 * | - | - | - | - | - | - | - |
| Flagellum | 17.1 ± 0.5 | 26.6 ± 1.0 * | 12.6 ± 0.2 | 23.5 ± 0.6 * | - | - | - | - | - | - | |
| Sensilla trichodeaI | 180.6 ± 5.4 | 410.2 ± 5.6 * | 3.8 ± 0.2 | 8.6 ± 0.2 * | - | - | - | - | Sharp | Yes | Pedicel |
| Sensilla trichodeaII | 13.2 ± 0.4 | 34.8 ± 1.8 * | 1.9 ± 0.1 | 3.2 ± 0.2 * | - | - | - | - | Sharp | Yes | Pedicel |
| Sensilla basiconicaI | 25.0 ± 1.1 | 30.7 ± 0.9 * | 8.8 ± 0.6 | 13.6 ± 0.6 * | - | - | - | - | Blunt | No | Pedicel and flagellum |
| Sensilla basiconicaII | 6.3 ± 0.4 | 7.2 ± 0.3 | 3.0 ± 0.2 | 3.3 ± 0.3 | - | - | - | - | Blunt | No | Flagellum |
| Sensilla chaetica | 7.1 ± 0.3 | 9.1 ± 0.7 | 2.0 ± 0.1 | 2.2 ± 0.1 * | - | - | - | - | Sharp | No | Pedicel and flagellum |
| Sensilla styloconicum | - | - | 5.0 ± 0.2 | 5.7 ± 0.1 * | 5.9 ± 0.3 | 6.6 ± 0.2 * | 10.7 ± 0.2 | 11.6 ± 0.2 * | Sharp | No | Flagellum |
| Sensilla cavity | - | - | 8.7 ± 0.3 | 19.2 ± 0.6 * | - | - | - | - | - | Yes | Pedicel |
| Antennae | Morphological Parameters | Female (µm) | Male (µm) |
|---|---|---|---|
| Scape | Length (µm) | 317.5 ± 9.1 | 313.3 ± 9.7 |
| Basal diameter (µm) | 291.2 ± 7.1 | 275.5 ± 6.9 | |
| Pedicel | Length (µm) | 219.5 ± 4.5 | 207.9 ± 7.8 |
| Basal diameter (µm) | 242.0 ± 6.0 | 236.0 ± 5.7 | |
| Flagellum | Subsegment length (µm) | 118.7 ± 3.3 | 114.7 ± 2.1 |
| Subsegment basal diameter (µm) | 140.6 ± 3.3 | 136.2 ± 3.0 | |
| Total | Antennal length (µm) | 7666.7 ± 124.5 | 7487.3 ± 112.5 |
| Type | Length (µm) | Basal Diameter (µm) | Width (µm) | Colum Diameter (µm) | Column Height (µm) | Peg Height (µm) | Top | Outer Wall | Socket | Distribution | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |||||
| Böhm bristlesI | 12.4 ± 0.3 | 13.5 ± 0.3 * | 2.6 ± 0.2 | 3.0 ± 0.1 | - | - | - | - | - | - | - | - | Sharp | Smooth | Yes | Scape and pedicel |
| Böhm bristlesII | 2.9 ± 0.3 | 2.9 ± 0.2 | - | - | - | - | - | - | - | - | - | - | Sharp | Smooth | No | Scape and pedicel |
| Sensilla trichodea | 30.7 ± 1.3 | 31.5 ± 1.4 | 3.1 ± 0.2 | 3.6 ± 0.2 | - | - | - | - | - | - | - | - | Blunt | Spiral lines, Porous | Yes | Flagellum |
| Sensilla basiconica | 21.2 ± 0.3 | 21.6 ± 0.6 | 4.5 ± 0.2 | 3.8 ± 0.2 * | - | - | - | - | - | - | - | - | Blunt | Longitudinal lines, Porous | Yes | Flagellum |
| Sensilla chaetica | 41.4 ± 1.4 | 49.7 ± 1.4 * | 4.6 ± 0.2 | 4.6 ± 0.1 | - | - | - | - | 4.6 ± 0.1 | 4.6 ± 0.1 | - | - | Blunt | Longitudinal lines | Yes | Flagellum |
| Sensilla coeloconica | - | - | 10.0 ± 0.3 | 10.3 ± 0.2 | - | - | - | - | - | - | - | - | Blunt | Longitudinal lines | Yes | Flagellum |
| Sensilla styloconicum | - | - | 5.8 ± 0.3 | 5.9 ± 0.3 | - | - | - | - | 20.4 ± 0.7 | 23.9 ± 0.4 * | 2.4 ± 0.1 | 2.5 ± 0.1 | Blunt | Reticular pattern | No | Flagellum |
| Sensilla cavity | - | - | 9.9 ± 0.2 | 9.6 ± 0.3 | - | - | - | - | - | - | - | - | - | Lines | Yes | Flagellum |
| Sensilla squamous | 71.7 ± 2.5 | 75.5 ± 1.2 | 3.5 ± 0.2 | 3.2 ± 0.1 | - | - | - | - | - | - | - | - | Sharp | Longitudinal lines | Yes | Uniform |
| Sensilla auricillica | 10.0 ± 0.3 | 10.8 ± 0.3 | - | - | - | - | - | - | - | - | - | - | Blunt | Longitudinal lines | Yes | Flagellum |
| Sensilla ligulate | 11.8 ± 0.3 * | 10.8 ± 0.3 | 5.2 ± 0.2 | 4.9 ± 0.1 | - | - | - | - | - | - | - | - | Blunt | Porous | Yes | Flagellum |
| Sensilla uniporous peg | - | - | 2.3 ± 0.3 | - | - | - | 1.1 ± 0.1 | - | 2.0 ± 0.2 | - | - | - | Blunt | Longitudinal lines, porous | Yes | Flagellum |
| Sensilla placodea | 13.6 ± 0.8 | 15.5 ± 0.5 | 4.5 ± 0.1 | 4.3 ± 0.2 | 2.5 ± 0.1 | 2.7 ± 0.1 | - | - | - | - | - | - | Blunt | Longitudinal lines, Porous | Yes | Flagellum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; He, P.; Liu, T.; Jing, X.; Zhang, S. Morphology and Distribution of Antennal Sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae and Adults. Diversity 2023, 15, 992. https://doi.org/10.3390/d15090992
Wang W, He P, Liu T, Jing X, Zhang S. Morphology and Distribution of Antennal Sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae and Adults. Diversity. 2023; 15(9):992. https://doi.org/10.3390/d15090992
Chicago/Turabian StyleWang, Wenwen, Pengyang He, Tongxian Liu, Xiangfeng Jing, and Shize Zhang. 2023. "Morphology and Distribution of Antennal Sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae and Adults" Diversity 15, no. 9: 992. https://doi.org/10.3390/d15090992
APA StyleWang, W., He, P., Liu, T., Jing, X., & Zhang, S. (2023). Morphology and Distribution of Antennal Sensilla on Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae and Adults. Diversity, 15(9), 992. https://doi.org/10.3390/d15090992