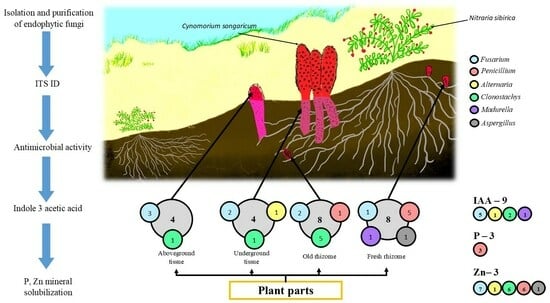
Identification, Antimicrobial and Plant Growth Promoting Activities of Endophytic Fungi Associated with Cynomorium songaricum Rupr., a Traditional Medicinal Plant in Mongolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Samples
2.2. Isolation of Endophytic Fungi
2.3. Identification of the Isolates
2.4. Antimicrobial Activity Test
2.5. Phosphate and Zinc Oxide Solubilization Assay
2.6. IAA Production Assay
3. Results
3.1. Isolation and Identification of Endophytic Fungi
3.2. Antimicrobial Activity
3.3. In Vitro Test for Plant Growth Promoting Traits of Endophytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CBD Convention on Biological Diversity. United Nations. 1992. Available online: https://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 11 March 2020).
- Law on Genetic Resources of Mongolia. 2021. Available online: https://legalinfo.mn/mn/detail?lawId=16390399395691 (accessed on 10 November 2023).
- Climate Change in Mongolia. Outputs from GCM. M. Available online: https://www.env.go.jp/content/900448010.pdf (accessed on 10 November 2023).
- Le Cocq, K.; Gurr, S.J.; Hirsch, P.R.; Mauchline, T.H. Exploitation of endophytes for sustainable agricultural intensification. Mol. Plant Pathol. 2017, 18, 469–473. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Sharma, A. Contemplating the role of zinc-solubilizing bacteria in crop biofortification: An approach for sustainable bioeconomy. Front. Agron. 2022, 4, 903321. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic fungi: From symbiosis to secondary metabolite communications or vice versa? Front. Plant Sci. 2021, 12, 791033. [Google Scholar] [CrossRef]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef]
- Jha, P.; Kaur, T.; Chhabra, I.; Panja, A.; Paul, S.; Kumar, V.; Malik, T. Endophytic fungi: Hidden treasure chest of antimicrobial metabolites interrelationship of endophytes and metabolites. Front. Microbiol. 2023, 14, 1227830. [Google Scholar] [CrossRef]
- Strobel, G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi 2018, 4, 57. [Google Scholar] [CrossRef]
- Tuvaanjav, S.; Shuqin, H.; Komata, M.; Ma, C.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. polysaccharides. J. Asian Nat. Prod. Res. 2016, 18, 159–171. [Google Scholar] [CrossRef]
- Cui, J.-L.; Gong, Y.; Xue, X.-Z.; Zhang, Y.-Y.; Wang, M.-L.; Wang, J.-H. A Phytochemical and Pharmacological Review on Cynomorium songaricum as Functional and Medicinal Food. Nat. Prod. Commun. 2018, 13, 501–510. [Google Scholar] [CrossRef]
- Ligaa, U.; Ninjil, N.; Davaadorj, T.; Lkhagvadorj, B.; Erdenetuya, N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine, 2nd ed.; JKC Printing: Ulaanbaatar, Mongolia, 2015; pp. 135–136. (In Mongolian) [Google Scholar]
- Cui, J.-L.; Vijayakumar, V.; Zhang, G. Partitioning of Fungal Endophyte Assemblages in Root-Parasitic Plant Cynomorium songaricum and Its Host Nitraria tangutorum. Front. Microbiol. 2018, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-L.; Gong, Y.; Vijayakumar, V.; Zhang, G.; Wang, M.-L.; Wang, J.-H.; Xue, X.-Z. Correlation in Chemical Metabolome and Endophytic Mycobiome in Cynomorium songaricum from Different Desert Locations in China. J. Agric. Food Chem. 2019, 67, 3554–3564. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.-M.; Zhang, Y.-Y.; Cui, J.-L.; Zhang, G. Species and geographic specificity between endophytic fungi and host supported by parasitic Cynomorium songaricum and its host Nitraria tangutorum distributed in desert. Arch. Microbiol. 2021, 203, 2511–2519. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Yang, J.H.; Liu, T.; Zhang, Z.W.; Mu, K.; Liang, F.H.; Liu, Y. The effect of salt stress on the growth of 3 species of Nitraria seedlings. IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012025. [Google Scholar] [CrossRef]
- Yuan, Y.-Y.; Sun, J.; Zhou, Y.-B.; Wang, J.; Deng, J.; Ye, R.-R.; Peng, M.; Lu, X.-F. Chemical Composition of Three Nitraria Species Fruits. Asian J. Chem. 2018, 30, 529–532. [Google Scholar] [CrossRef]
- Heiner, M.; Galbadrakh, D.; Kiesecker, J. Shifting winds in the Mongolian Gobi Desert: Nature and traditions face the modern era. In Encyclopedia of the World’s Biomes; Goldstein, M.I., DellaSala, D.A., Eds.; Elsevier: Oxford, UK, 2020; pp. 78–84. [Google Scholar]
- Le, T.T.M.; Hoang, A.T.H.; Le, T.T.B.; Vo, T.T.B.; Van Quyen, D.; Chu, H.H. Isolation of endophytic fungi and screening of Huperzine A–producing fungus from Huperzia serrata in Vietnam. Sci. Rep. 2019, 9, 16152. [Google Scholar] [CrossRef]
- Barman, A.; Nath, A.; Thakur, D. Identification and characterization of fungi associated with blister blight lesions of tea (Camellia sinensis L. Kuntze) isolated from Meghalaya, India. Microbiol. Res. 2020, 240, 126561. [Google Scholar] [CrossRef]
- Hechmi, N.; Bosso, L.; El-Bassi, L.; Scelza, R.; Testa, A.; Jedidi, N.; Rao, M.A. Depletion of pentachlorophenol in soil microcosms with Byssochlamys nivea and Scopulariopsis brumptii as detoxification agents. Chemosphere 2016, 165, 547–554. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ibrahim, D.; Lee, C.C.; Sheh-Hong, L. Antimicrobial Activity of Endophytic Fungi Isolated from Swietenia macrophylla Leaves. Nat. Prod. Commun. 2014, 9, 247–250. [Google Scholar] [CrossRef]
- Bosso, L.; Scelza, R.; Varlese, R.; Meca, G.; Testa, A.; Rao, M.A.; Cristinzio, G. Assessing the effectiveness of Byssochlamys nivea and Scopulariopsis brumptii in pentachlorophenol removal and biological control of two Phytophthora species. Fungal Biol. 2016, 120, 645–653. [Google Scholar] [CrossRef]
- Kumar, C.S.; Jacob, T.; Devasahayam, S.; Thomas, S.; Geethu, C. Multifarious plant growth promotion by an entomopathogenic fungus Lecanicillium psalliotae. Microbiol. Res. 2018, 207, 153–160. [Google Scholar] [CrossRef]
- Rajini, S.B.; Nandhini, M.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol. 2020, 69, 642–654. [Google Scholar] [CrossRef]
- Saikkonen, K. Forest structure and fungal endophytes. Fungal Biol. Rev. 2007, 21, 67–74. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Oshita, Y.; Yasuda, M.; Kanasugi, M.; Matsuura, E.; Xu, Q.; Okazaki, S. Host Specificity of Endophytic Fungi from Stem Tissue of Nature Farming Tomato (Solanum lycopersicum Mill.) in Japan. Agronomy 2020, 10, 1019. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Wylie, S.J. Host Specificity of Endophytic Mycobiota of Wild Nicotiana Plants from Arid Regions of Northern Australia. Microb. Ecol. 2018, 75, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Miao, L.; Li, H.; Lin, A.; Song, F.; Zhang, P. Illumina-based analysis yields new insights into the diversity and composition of endophytic fungi in cultivated Huperzia serrata. PLoS ONE 2020, 15, e0242258. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.C.; Yu, S.H. Endophytic Fungi from Medicinal Plants in Korea; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Guevara-Araya, M.J.; Vilo, C.; Urzúa, A.; González-Teuber, M. Differences in community composition of endophytic fungi between above- and below-ground tissues of Aristolochia chilensis in an arid ecosystem. Rev. Chil. Hist. Nat. 2020, 93, 3. [Google Scholar] [CrossRef]
- Toghueo, R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Gams, W.; Diederich, P.; Põldmaa, K. Fungicolos Fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Muller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: Burlington, NJ, USA, 2004; Volume 465, pp. 343–392. [Google Scholar] [CrossRef]
- Jensen, D.F.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys rosea to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 429–472. [Google Scholar] [CrossRef]
- Han, P.; Zhang, X.; Xu, D.; Zhang, B.; Lai, D.; Zhou, L. Metabolites from Clonostachys Fungi and Their Biological Activities. J. Fungi 2020, 6, 229. [Google Scholar] [CrossRef]
- Kapeua-Ndacnou, M.; de Abreu, L.M.; de Macedo, D.M.; da Nóbrega, T.F.; Pereira, C.M.; Evans, H.C.; Barreto, R.W. Assessing the Biocontrol Potential of Clonostachys Species Isolated as Endophytes from Coffea Species and as Mycoparasites of Hemileia Rusts of Coffee in Africa. J. Fungi 2023, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Vilo, C.; Bascuñán-Godoy, L. Molecular characterization of endophytic fungi associated with the roots of Chenopodium quinoa inhabiting the Atacama Desert. Genom. Data 2017, 11, 109–112. [Google Scholar] [CrossRef]
- González-Teuber, M.; Urzúa, A.; Morales, A.; Ibáñez, C.; Bascuñán-Godoy, L. Benefits of a root fungal endophyte on physiological processes and growth of the vulnerable legume tree Prosopis chilensis (Fabaceae). J. Plant Ecol. 2019, 12, 264–271. [Google Scholar] [CrossRef]
- González-Teuber, M.; Urzua, A.; Plaza, P.; Bascuñán-Godoy, L. Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol. 2018, 219, 231–240. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, X.-R.; Wu, X.-Q.; Li, G.-E.; Wang, Z.; Li, D.-W. The phosphate-solubilising ability of Penicilium guanacastense and its effects on the growth of Pinus massoniana in phosphate limiting conditions. Biol. Open 2019, 8, bio046797. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Warren, R.A.; Harvey, P.R.; Ryder, M.H. Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol. Fertil. Soils 2004, 40, 36–43. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.-W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.-F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of Phosphate-Solubilizing Fungi from Air and Soil in Yunnan, China: Four Novel Species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Devi, D.; Gupta, S.B.; Mishra, B.K.; Verma, N.P. Isolation and identification of zinc solubilizing fungal isolates from cumin of semi-arid region of Rajasthan. Pharma Innov. 2022, 11, 1036–1040. [Google Scholar]
- de Hoog, G.S.; van Diepeningen, A.D.; Mahgoub, E.-S.; van de Sande, W.W.J. New Species of Madurella, Causative Agents of Black-Grain Mycetoma. J. Clin. Microbiol. 2012, 50, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, H.; Kavya, S.; Pooja, K.; Sneha, L.; Arer, V.O. An isolation, identification and diversity of endophytic fungi from Catharanthus roseus and screening for their L-asparaginase activity. Int. J. Environ. Ecol. Fam. Urban Stud. 2018, 8, 7–18. [Google Scholar]
- Hapida, Y.; Elfita, E.; Widjajanti, H.; Salni, S. Biodiversity and antibacterial activity of endophytic fungi isolated from jambu bol (Syzygium malaccense). Biodiversitas J. Biol. Divers. 2021, 22, 5668–5677. [Google Scholar] [CrossRef]
- Orole, O.O.; Adejumo, T.O.; Link, T.; Voegele, R.T. Molecular identification of endophytes from maize roots and their biocontrol potential against toxigenic fungi of Nigerian maize. Sci. Prog. 2023, 106, 368504231186514. [Google Scholar] [CrossRef]




| Strain | Accession No | Plant Part | Species with Most Homologous Sequence (Accession No) | Similarity % |
|---|---|---|---|---|
| P26-H1-1 | LC769420 | Aboveground | Fusarium equiseti CB33-4 (MT558601) | 99.81 |
| P26-H1-3 | LC769421 | Aboveground | Clonostachys rosea MR44 (KY320599) | 99.65 |
| P26-H2-1 | LC769422 | Aboveground | Fusarium solani GBC-Fungus 27 (MN077430) | 100 |
| P26-H2-2 | LC769423 | Aboveground | Fusarium solani N-49-1 (MT560378) | 100 |
| P26-R1-1 | LC769424 | Underground | Fusarium equiseti NL-374-D (OQ561206) | 99.63 |
| P26-R1-3 | LC769425 | Underground | Alternaria sp. INM5 (KY781740) | 99.30 |
| P26-R2-1 | LC769426 | Underground | Fusarium solani N-13-2 (MT560338) | 100 |
| P26-R2-2 | LC769427 | Underground | Clonostachys rosea MR44 (KY320599) | 99.13 |
| P26-ZN1-1 | LC769428 | Fresh rhizome | Penicillium chrysogenum MZC-0 (MN069559) | 99.66 |
| P26-ZN1-2 | LC769429 | Fresh rhizome | Madurella fahalii 332- pus (OQ421454) | 98.96 |
| P26-ZN1-3 | LC769430 | Fresh rhizome | Aspergillus tabacinus fung8 (MT635280) | 100 |
| P26-ZN2-2 | LC769431 | Fresh rhizome | Penicillium chrysogenum MZC-0 (MN069559) | 99.83 |
| P26-ZN2-3 | LC769432 | Fresh rhizome | Penicillium roseopurpureum IHEM:28005 (OU989457) | 99.83 |
| P26-ZN2-4 | LC769433 | Fresh rhizome | Fusarium sp. GFR18 (MT447523) | 100 |
| P26-ZN2-5 | LC769434 | Fresh rhizome | Penicillium vinaceum 533 (DQ681340) | 100 |
| P26-ZN2-6 | LC769435 | Fresh rhizome | Penicillium roseopurpureum G5-2 (MN206951) | 100 |
| P26-ZO1-1 | LC769436 | Old rhizome | Penicillium sp. FP-027-A7 (MH102087) | 99.49 |
| P26-ZO1-3 | LC769437 | Old rhizome | Clonostachys rosea daef27 (MH550497) | 99.12 |
| P26-ZO1-4 | LC663164 | Old rhizome | Clonostachys rosea Potato root (MT448899) | 100 |
| P26-ZO1-5 | LC769438 | Old rhizome | Clonostachys sp. 1R1D (OR365747) | 99.82 |
| P26-ZO1-6 | LC769439 | Old rhizome | Fusarium sp. GFR18 (MT447523) | 99.82 |
| P26-ZO2-1 | LC769440 | Old rhizome | Fusarium proliferatum CBB-6 (MT560216) | 100 |
| P26-ZO2-2 | LC769441 | Old rhizome | Clonostachys rosea MR44 (KY320599) | 99.83 |
| P26-ZO2-3 | LC769442 | Old rhizome | Clonostachys rosea N25 (MH259861) | 100 |
| Strain | Taxa | Diameter of the Inhibitory Zone (mm) | ||||
|---|---|---|---|---|---|---|
| Escherichia coli | Bacillus subtilis | Staphylococcus aureus | Candida albicans | Aspergillus niger | ||
| P26-H1-1 | Fusarium equiseti | - | 15.5 ± 0.7 | 12 ± 0 | - | - |
| P26-H1-3 | Clonostachys rosea | - | 10.5 ± 0.7 | - | - | 15.5 ± 0.7 |
| P26-H2-1 | Fusarium proliferatum | - | 11.5 ± 1.4 | 13 ± 0.7 | - | - |
| P26-H2-2 | Fusarium solani | - | - | 10.5 ± 0.7 | - | 23 ± 2.8 |
| P26-R1-1 | Fusarium equiseti | - | 15.5 ± 0.7 | 13.5 ± 0.7 | - | - |
| P26-R2-1 | Fusarium solani | - | - | - | - | 22.5 ± 0.7 |
| P26-R2-2 | Clonostachys rosea | - | - | 7.5 ± 2.1 | - | - |
| P26-ZN1-2 | Madurella fahalii | 7.5 ± 0.7 | 9.5 ± 2.1 | 15.5 ± 0.7 | - | - |
| P26-ZN1-3 | Aspergillus amoenus | - | 8 ± 1.4 | 7 ± 0 | - | - |
| P26-ZO1-3 | Clonostachys rosea | - | 15 ± 0 | 18.5 ± 0.7 | - | 13.5 ± 2.1 |
| P26-ZO1-4 | Clonostachys rosea | - | 9 ± 4.2 | 9.5 ± 0.7 | - | 12.5 ± 2.1 |
| P26-ZO1-5 | Clonostachys rosea | - | 13.5 ± 0.7 | 16.5 ± 0.7 | - | 14 ± 1.4 |
| P26-ZO1-6 | Fusarium tonkinense | - | 11.5 ± 0.7 | 17.5 ± 0.7 | - | 12 ± 0 |
| P26-ZO2-2 | Clonostachys rosea | - | 12 ± 0 | 15.5 ± 2.1 | - | - |
| P26-ZO2-3 | Clonostachys rosea | - | 13.5 ± 0.7 | 16 ± 0 | - | 13 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jigjiddorj, E.-A.; Maidarjav, A.; Byambasuren, B.; Nyamgerel, D. Identification, Antimicrobial and Plant Growth Promoting Activities of Endophytic Fungi Associated with Cynomorium songaricum Rupr., a Traditional Medicinal Plant in Mongolia. Diversity 2024, 16, 122. https://doi.org/10.3390/d16020122
Jigjiddorj E-A, Maidarjav A, Byambasuren B, Nyamgerel D. Identification, Antimicrobial and Plant Growth Promoting Activities of Endophytic Fungi Associated with Cynomorium songaricum Rupr., a Traditional Medicinal Plant in Mongolia. Diversity. 2024; 16(2):122. https://doi.org/10.3390/d16020122
Chicago/Turabian StyleJigjiddorj, Enkh-Amgalan, Amarbayasgalan Maidarjav, Bumtsend Byambasuren, and Daritsogzol Nyamgerel. 2024. "Identification, Antimicrobial and Plant Growth Promoting Activities of Endophytic Fungi Associated with Cynomorium songaricum Rupr., a Traditional Medicinal Plant in Mongolia" Diversity 16, no. 2: 122. https://doi.org/10.3390/d16020122
APA StyleJigjiddorj, E.-A., Maidarjav, A., Byambasuren, B., & Nyamgerel, D. (2024). Identification, Antimicrobial and Plant Growth Promoting Activities of Endophytic Fungi Associated with Cynomorium songaricum Rupr., a Traditional Medicinal Plant in Mongolia. Diversity, 16(2), 122. https://doi.org/10.3390/d16020122