Acute Response in vivo of a Fiber-Optic Sensor for Continuous Glucose Monitoring from Canine Studies on Point Accuracy
Abstract
:1. Introduction
2. Experimental Section
2.1. Canine Subjects
2.2. Anesthesia
2.3. Sensor Placement and Spacial Arrangements
2.4. In Vitro Sensor Calibration
2.5. Initiation and Processing of Monitoring
3. Results and Discussion
3.1. Canine Subjects
3.2. Canine Studys #1–3
3.3. Canine Study #4
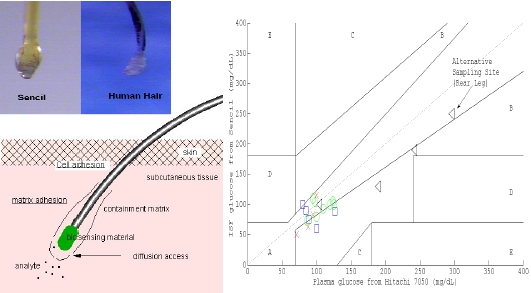
3.4. Accuracy of Agreement between Sencil Detections and Plasma Reference for Clinical Application
4. Conclusions
Acknowledgments
References
- Pickup, JC. In vivo glucose monitoring: Sense and sensorobility. Diabetes Care 1993, 16, 535–539. [Google Scholar]
- Wilson, GS; Reach, G. Diabetes and glucose sensors. Anal. Chem 1992, 64, 381A–384A. [Google Scholar]
- Diabetes Control and Complication Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med 1993, 329, 977–986. [Google Scholar]
- UK Prospective Diabetes Study Group (UKPDS 33). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet 1998, 352, 837–853. [Google Scholar]
- Wentholt, I; Hoekstra, J; DeVries, J. A critical appraisal of the continuous error grid analysis. Diabetes Care 2006, 29, 1805–1811. [Google Scholar]
- Steil, GM; Panteleon, AE; Rebrin, K. Close-loop insulin delivery—The path to physiological glucose control. Advan. Drug Delivery Rev 2004, 56, 125–144. [Google Scholar]
- Clarke, WL. The original Clarke error grid analysis (EGA). Diabetes Technol. Ther 2005, 7, 776–779. [Google Scholar]
- Steil, GM; Rebrin, K; Mastrototaro, JJ. Metabolic modeling and the close-loop insulin delivery problem. Diabetes Res. Clin. Pract 2006, 74, S183–S186. [Google Scholar]
- Steil, GM; Rebrin, K; Janowski, R; Darwin, C; Saad, MF. Modeling β-cell secretion—Implications for closed-loop glucose homeostasis. Diabetes Technol. Ther 2003, 5, 953–964. [Google Scholar]
- Liao, KC; Hogen-Esch, T; Richmond, FJ; Marcu, L; Clifton, W; Loeb, GE. Percutaneous fiber-optic sensor for chronic glucose monitoring in vivo. Biosensor. Bioelectron 2008, 23, 1458–1465. [Google Scholar]
- Liao, KC; Hogen-Esch, T; Richmond, FJ; Marcu, L; Loeb, GE. Design and Fabrication of Disposable, Percutaneous Chemical Sensors. Proceedings of Optical Fibers and Sensors for Medical Application V, SPIE, Bellingham, WA, USA, July 2005; 5691, pp. 129–145.
- Garg, S; Zisser, H; Schwartz, S; Bailey, T; Kaplan, R; Ellis, S; Jovanovic, L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: A randomized controlled trial. Diabetes Care 2006, 29, 44–50. [Google Scholar]
- Garg, SK; Potts, RO; Ackerman, NR; Fermi, SJ; Tamada, JA; Chase, HP. Correlation of fingerstick blood glucose measurements with GlucoWatch biographer glucose result in young subjects with type 1 diabetes. Diabetes Care 1999, 130, 1708–1714. [Google Scholar]
- Shichri, M; Sakakida, M; Nishida, K; Shioda, S. Enhanced, simplified glucose sensors: Long-term clinical application of wearable artificial endocrine pancreas. Artific. Organ 1998, 22, 32–42. [Google Scholar]
- Bindra, DS; Zhang, Y; Wilson, GS; Sternberg, R; Thevenot, DR; Moatti, D; Reach, G. Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring. Anal. Chem 1991, 63, 1692–1696. [Google Scholar]
- Boyne, MS; Silver, DM; Kaplan, J; Saudek, CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003, 52, 2790–2794. [Google Scholar]
- Aussedat, B; Dupire-Angel, M; Gifford, R; Klein, JC; Wilson, GS; Reach, G. Interstitial glucose concentration and glycemia: Implications for continuous subcutaneous glucose monitoring. Amer. J. Physiol-Endocr. Metab 2000, 278, E716–728. [Google Scholar]
- Kulcu, E; Potts, RO; Tamada, JA; Lesho, MJ; Reach, G. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care 2003, 26, 2405–2409. [Google Scholar]
- Rebrin, K; Steil, GM. Can interstitial glucose assessment replace blood glucose measurement? Diabetes Technol. Ther 2000, 2, 461–471. [Google Scholar]
- Rebrin, K; Steil, GM; van Antwerp, WP; Mastrototaro, JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: Implications for continuous monitoring. Am. J. Physiol-Endocrinol. Metab 1999, 277, E561–E571. [Google Scholar]
- Panteleon, AE; Rebrin, K; Steil, GM. The role of the independent variable to glucose sensor calibration. Diabetes Technol. Ther 2003, 5, 401–410. [Google Scholar]
- Hecht, E. The propagation of light. In Optics, 4th ed; Addison Wesley: San Francisco, CA, USA, 2002; pp. 86–148. [Google Scholar]
- Labow, RS; Erfle, DJ; Santerre, JP. Neutrophil-mediated degradation of segmented polyurethanes. Biomaterials 1995, 16, 51–59. [Google Scholar]
- Heninger, N; Woderer, S; Kloetzer, HM; Staib, A; Gillen, R; Li, L; Yu, X; Norbert, G; Kraenzlin, B; Pill, J. Tissue response to subcutaneous implantation of glucose-oxidase-based glucose sensor in rats. Biosensor. Bioelectron 2007, 233, 26–34. [Google Scholar]
- Klueh, U; Kaur, M; Qiao, Y; Kreutzer, DL. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials 2010, 31, 4540–4551. [Google Scholar]
- Guerci, B; Benichu, M; Floriot, M; Jellimann, S; Bohme, P; Drouin, P; Durain, D. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analog. Diabetes Care 2003, 26, 582–589. [Google Scholar]








© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, K.-C.; Chang, S.-C.; Chiu, C.-Y.; Chou, Y.-H. Acute Response in vivo of a Fiber-Optic Sensor for Continuous Glucose Monitoring from Canine Studies on Point Accuracy. Sensors 2010, 10, 7789-7802. https://doi.org/10.3390/s100807789
Liao K-C, Chang S-C, Chiu C-Y, Chou Y-H. Acute Response in vivo of a Fiber-Optic Sensor for Continuous Glucose Monitoring from Canine Studies on Point Accuracy. Sensors. 2010; 10(8):7789-7802. https://doi.org/10.3390/s100807789
Chicago/Turabian StyleLiao, Kuo-Chih, Shih-Chieh Chang, Cheng-Yang Chiu, and Yu-Hsiang Chou. 2010. "Acute Response in vivo of a Fiber-Optic Sensor for Continuous Glucose Monitoring from Canine Studies on Point Accuracy" Sensors 10, no. 8: 7789-7802. https://doi.org/10.3390/s100807789
APA StyleLiao, K. -C., Chang, S. -C., Chiu, C. -Y., & Chou, Y. -H. (2010). Acute Response in vivo of a Fiber-Optic Sensor for Continuous Glucose Monitoring from Canine Studies on Point Accuracy. Sensors, 10(8), 7789-7802. https://doi.org/10.3390/s100807789