An Easily Fabricated Electrochemical Sensor Based on a Graphene-Modified Glassy Carbon Electrode for Determination of Octopamine and Tyramine
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Instruments
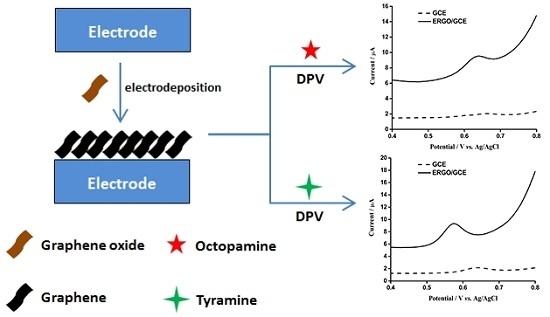
2.3. Preparation of ERGO/GCE
2.4. Analytical Procedure
3. Results and Discussion
3.1. Electrodeposition of Graphene Nanosheets
3.2. Electrochemical Detection of OA and TA with ERGO/GCE
3.3. The Effect of CV Scanning Rate and pH on the Electrochemical Oxidations of OA and TA on ERGO/GCE
3.4. Optimization of Key Parameters for Electrochemical Oxidations of OA and TA
3.5. Reproducibility, Linear Range and Limit of Detection
3.6. Interference Studies
3.7. Stability
3.8. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernández-Jover, T.; Izquierdo-Pulido, M.; Veciana-Nogués, M.T.; Mariné-Font, A.; Vidal-Carou, M.C. Biogenic Amine and Polyamine Contents in Meat and Meat Products. J. Agric. Food Chem. 1997, 45, 2098–2102. [Google Scholar] [CrossRef]
- Bulushi, I.A.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Toro-Funes, N.; Bosch-Fuste, J.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Biologically active amines in fermented and non-fermented commercial soybean products from the Spanish market. Food Chem. 2015, 173, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Liu, S.; Yu, J.; Lian, W.; Huang, J. Electrochemical sensor based on molecularly imprinted film at polypyrrole-sulfonated graphene/hyaluronic acid-multiwalled carbon nanotubes modified electrode for determination of tryptamine. Biosens. Bioelectron. 2012, 31, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.K. Biogenic and volatile amine-related qualities of three popular fish species sold at Kuwait fish markets. Food Chem. 2008, 107, 761–767. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P.; Henri-Dubernet, S.; Guéguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Leitão, M.C.; San Romão, M.V. Biogenic amines in wines: Influence of oenological factors. Food Chem. 2008, 107, 853–860. [Google Scholar] [CrossRef]
- Ntzimani, A.G.; Paleologos, E.K.; Savvaidis, I.N.; Kontominas, M.G. Formation of biogenic amines and relation to microbial flora and sensory changes in smoked turkey breast fillets stored under various packaging conditions at 4 °C. Food Microbiol. 2008, 25, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Bomke, S.; Seiwert, B.; Dudek, L.; Effkemann, S.; Karst, U. Determination of biogenic amines in food samples using derivatization followed by liquid chromatography/mass spectrometry. Anal. Bioanal. Chem. 2009, 393, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Viana, C.; Zemolin, G.M.; Lima, F.O.; de Carvalho, L.M.; Bottoli, C.B.G.; Limberger, R.P. High-performance liquid chromatographic analysis of biogenic amines in pharmaceutical products containing Citrus aurantium. Food Addit. Contam. Part A 2013, 30, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, I.; Arain, G.M.; Khuhawar, M.Y. Liquid Chromatographic Determination of Neurotransmitter Serotonin from Human Cerebrospinal Fluid Using 2-Hydroxynaphtaldehyde as Derivatizing Reagent. Asian J. Chem. 2013, 25, 9452–9456. [Google Scholar]
- Bruckner, H.; Flassig, S.; Kirschbaum, J. Determination of biogenic amines in infusions of tea (Camellia sinensis) by HPLC after derivatization with 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl). Amino Acids 2012, 42, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Jin, C.-X.; Song, S.-L.; Wei, C.-Y.; Liu, Y.-M.; Li, J. Development of an ionic liquid-based ultrasonic-assisted liquid-liquid microextraction method for sensitive determination of biogenic amines: Application to the analysis of octopamine, tyramine and phenethylamine in beer samples. J. Chromatogr. B 2011, 879, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Mazzucco, E.; Gennaro, M.C.; Marengo, E. Simultaneous determination of sixteen underivatized biogenic amines in human urine by HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Moratalla, M.L.; Bosch-Fusté, J.; Lavizzari, T.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Validation of an ultra high pressure liquid chromatographic method for the determination of biologically active amines in food. J. Chromatogr. A 2009, 1216, 7715–7720. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.K.; Fiechter, G.; Fischer, E. A new ultra-pressure liquid chromatography method for the determination of biogenic amines in cheese. J. Chromatogr. A 2010, 1217, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Montealegre, C.; Crego, A.L.; Marina, M.L. Recent contributions of capillary electrophoresis to neuroscience. Trends Anal. Chem. 2015, 67, 82–99. [Google Scholar] [CrossRef]
- Berglund, E.C.; Kuklinski, N.J.; Karagündüz, E.; Ucar, K.; Hanrieder, J.; Ewing, A.G. Freeze-Drying as Sample Preparation for Micellar Electrokinetic Capillary Chromatography-Electrochemical Separations of Neurochemicals in Drosophila Brains. Anal. Chem. 2013, 85, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, N.J.; Berglund, E.C.; Engelbrektsson, J.; Ewing, A.G. Biogenic Amines in Microdissected Brain Regions of Drosophila Melanogaster Measured with Mice Liar Electrokinetic Capillary Chromatography-Electrochemical Detection. Anal. Chem. 2010, 82, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Dailey, C.; Garnier, N.; Rubakhin, S.; Sweedler, J. Automated method for analysis of tryptophan and tyrosine metabolites using capillary electrophoresis with native fluorescence detection. Anal. Bioanal. Chem. 2013, 405, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Bacaloni, A.; Insogna, S.; Sancini, A.; Ciarrocca, M.; Sinibaldi, F. Sensitive profiling of biogenic amines in human urine by capillary electrophoresis with field amplified sample injection. Biomed. Chromatogr. 2013, 27, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Breadmore, M.C. Capillary and microchip electrophoresis: Challenging the common conceptions. J. Chromatogr. A 2012, 1221, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Bóka, B.; Adányi, N.; Virág, D.; Sebela, M.; Kiss, A. Spoilage Detection with Biogenic Amine Biosensors, Comparison of Different Enzyme Electrodes. Electroanalysis 2012, 24, 181–186. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. Amperometric biosensor based on polypyrrole and tyrosinase for the detection of tyramine in food samples. Sens. Actuators B Chem. 2013, 178, 40–46. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Baghayeri, M.; Amiri-Aref, M. Application of a glassy carbon electrode modified with functionalized multi-walled carbon nanotubes as a sensor device for simultaneous determination of acetaminophen and tyramine. Anal. Methods 2012, 4, 1579–1587. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Amiri-Aref, M.; Baghayeri, M. Electrodeposition of quercetin at a multi-walled carbon nanotubes modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of levodopa, uric acid and tyramine. Sens. Actuators B Chem. 2012, 166, 508–518. [Google Scholar] [CrossRef]
- Huang, J.D.; Xing, X.R.; Zhang, X.M.; He, X.R.; Lin, Q.; Lian, W.J.; Zhu, H. A molecularly imprinted electrochemical sensor based on multiwalled carbon nanotube-gold nanoparticle composites and chitosan for the detection of tyramine. Food Res. Int. 2011, 44, 276–281. [Google Scholar] [CrossRef]
- Cooper, S.E.; Venton, B.J. Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Anal. Bioanal. Chem. 2009, 394, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.R.; Anderson, S.E.; Privman, E.; Bau, H.H.; Venton, B.J. Carbon Nanopipette Electrodes for Dopamine Detection in Drosophila. Anal. Chem. 2015, 87, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L.; McDermott, M.T. Comment on electrochemical kinetics at ordered graphite electrodes. Anal. Chem. 2012, 84, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, J.K.; McDermott, M.T. Formation of multilayers on glassy carbon electrodes via the reduction of diazonium salts. Langmuir 2001, 17, 5947–5951. [Google Scholar] [CrossRef]
- Kariuki, J.K.; McDermott, M.T. Nucleation and Growth of Functionalized Aryl Films on Graphite Electrodes. Langmuir 1999, 15, 6534–6540. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nature Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Yang, Y.; Peng, Z.; Li, J.; Mei, T.; Wang, J.; Hao, M.; Chen, Y.; Xiong, W.; et al. A facile strategy to three-dimensional Pd@Pt core-shell nanoflowers supported on graphene nanosheets as an enhanced nanoelectrocatalyst for methanol oxidation. Chem. Commun. 2015, 51, 10490–10493. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, X.Z.; Wang, Y.H.; Ju, X.M.; Xu, L.; Li, G.J.; Sun, Z.F. Application of graphene-SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochim. Acta 2013, 87, 317–322. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahighi, R. Toward Single-DNA Electrochemical Biosensing by Graphene Nanowalls. ACS Nano 2012, 6, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Wang, L.-H.; Li, X.; Huang, W. Layer-controllable WS2-reduced graphene oxide hybrid nanosheets with high electrocatalytic activity for hydrogen evolution. Nanoscale 2015, 7, 10391–10397. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Z.; Dong, Y.; Li, D. Electrochemical Behavior of O-Nitrophenol at Hexagonal Mesoporous Silica Modified Carbon Paste Electrodes. Electroanalysis 2009, 21, 853–858. [Google Scholar] [CrossRef]
- Łuczak, T. Electrochemical behaviour of benzylamine, 2-phenylethylamine and 4-hydroxyphenylethylamine at gold. A comparative study. J. Appl. Electrochem. 2007, 38, 43–50. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, S.; Xu, F.; Chen, S.; Jia, J.; Tan, H.; Hou, H.; Song, Y. Electrochemical Sensing and Biosensing Platform Based on Biomass-Derived Macroporous Carbon Materials. Anal. Chem. 2014, 86, 1414–1421. [Google Scholar] [CrossRef] [PubMed]









| Electrode | Technique | Linear Range (μM) | LOD (S/N = 3) | Refs. |
|---|---|---|---|---|
| Monoamine oxidase A/graphite electrode | Amperometry | TA: 10–500 | 2 μM | [23] |
| Tyrosinase/polypyrrole/Pt electrode | Amperometry | TA: 4–80 | 0.547 μM | [24] |
| Tyrosinase/Carboxyl functionalised SWCNTs | Amperometry | TA: 5–180 | 0.62 μM | [25] |
| Carboxyl functionalised MWCNT/GCE | DPV | TA: 1–17 and 17–85 | TA: 0.42 μM | [26] |
| Quercetin/carboxyl functionalised MWCNT/GCE | DPV | TA: 0.7–75 | TA: 0.647 μM | [27] |
| Carbon nanofiber | Fast CV | OA and TA: 0.2–5 | OA: 30 nM | [29] |
| TA: 18 nM | ||||
| MWCNT-AuNP/chitosan/GCE | Amperometry | TA: 0.108–10 | TA: 57 nM | [28] |
| ERGO/GCE | DPV | OA: 0.5–40 | OA: 100 Nm | This work |
| TA: 0.1–25 | TA: 30 nM |
| Analyte | Sample | Add (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| OA | 1 | 5 | 4.925 | 98.5 | 5.9 |
| 2 | 30 | 31.41 | 104.7 | 6.1 | |
| TA | 1 | 5 | 5.11 | 102.2 | 5.6 |
| 2 | 10 | 10.31 | 103.1 | 6.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, M.; Wei, Q.; Gao, Y.; Guo, L.; Al-Ghanim, K.A.; Mahboob, S.; Zhang, X. An Easily Fabricated Electrochemical Sensor Based on a Graphene-Modified Glassy Carbon Electrode for Determination of Octopamine and Tyramine. Sensors 2016, 16, 535. https://doi.org/10.3390/s16040535
Zhang Y, Zhang M, Wei Q, Gao Y, Guo L, Al-Ghanim KA, Mahboob S, Zhang X. An Easily Fabricated Electrochemical Sensor Based on a Graphene-Modified Glassy Carbon Electrode for Determination of Octopamine and Tyramine. Sensors. 2016; 16(4):535. https://doi.org/10.3390/s16040535
Chicago/Turabian StyleZhang, Yang, Meiqin Zhang, Qianhui Wei, Yongjie Gao, Lijuan Guo, Khalid A. Al-Ghanim, Shahid Mahboob, and Xueji Zhang. 2016. "An Easily Fabricated Electrochemical Sensor Based on a Graphene-Modified Glassy Carbon Electrode for Determination of Octopamine and Tyramine" Sensors 16, no. 4: 535. https://doi.org/10.3390/s16040535
APA StyleZhang, Y., Zhang, M., Wei, Q., Gao, Y., Guo, L., Al-Ghanim, K. A., Mahboob, S., & Zhang, X. (2016). An Easily Fabricated Electrochemical Sensor Based on a Graphene-Modified Glassy Carbon Electrode for Determination of Octopamine and Tyramine. Sensors, 16(4), 535. https://doi.org/10.3390/s16040535