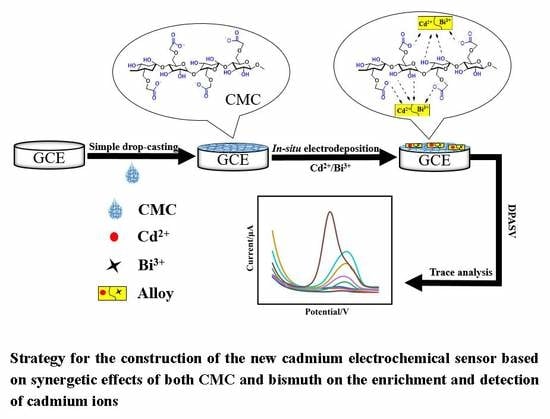
Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Preparation of the Working Electrode
2.4. Electrochemical Measurements
2.5. Detection of Cadmium Ion in Natural Samples
3. Results and Discussion
3.1. Investigation on the CMC, CMC-Cd and CMC-Cd-Bi by FT-IR
3.2. Investigation on the Synergetic Effects of Both CMC and Bismuth on the Enrichment of Cadmium by SEM and EDS
3.3. Construction and Investigation of the Proposed Cadmium Sensor
3.4. Optimization of Experimental Conditions
3.4.1. The pH Value of the Buffer Solution
3.4.2. Accumulation Time
3.4.3. Deposition Potential
3.4.4. The Concentration of Sodium Carboxymethyl Cellulose (CMC)
3.4.5. The Concentration of Bismuth Ion Solution (Bi3+)
3.5. Sensitivity of the Proposed Cadmium Sensor
3.6. Interference Study
3.7. Reproducibility and Repeatability of the Proposed Sensor
3.8. Analysis of Tap Water Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic (III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd (II) and Pb (II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/Nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Hutton, M. Sources of cadmium in the environment. Ecotoxicol. Environ. Saf. 1983, 7, 9–24. [Google Scholar] [CrossRef]
- Noh, Y.; Jo, E.J.; Mun, H.; Ahn, Y.D.; Kim, M.G. Homogeneous and selective detection of cadmium ions by forming fluorescent cadmium-protein nanoclusters. Chemosphere 2017, 174, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, S.; Silva, M.F.; Gásquez, J.A.; Olsina, R.A.; Martinez, L.D. On-line preconcentration/determination of cadmium in drinking water on activated carbon using 8-hydroxyquinoline in a flow injection system coupled to an inductively coupled plasma optical emission spectrometer. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 43–50. [Google Scholar] [CrossRef]
- Flick, D.F.; Kraybill, H.F.; Dlmitroff, J.M. Toxic effects of cadmium: A review. Environ. Res. 1971, 4, 71–85. [Google Scholar] [CrossRef]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 1–18. [Google Scholar]
- Mudila, H.; Prasher, P.; Kumar, M.; Kapoor, H.; Kumar, A.; Zaidi, M.G.H.; Verma, A. An insight into Cadmium poisoning and its removal from aqueous sources by Graphene Adsorbents. Int. J. Environ. Health Res. 2019, 29, 1–21. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Bond, A.; Wallace, G. Liquid chromatography with electrochemical and or spectrophotometric detection for automated determination of lead, cadmium, mercury, cobalt, nickel and copper. Anal. Chem. 1984, 56, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Arpadjan, S.; Celik, G.; Taskesen, S.; Gucer, S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008, 46, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, M.D.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Direct determination of toxic trace metals in honey and sugars using inductively coupled plasma atomic emission spectrometry. Talanta 2005, 65, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Ning, J.; Liu, L.; Luo, X.; Wang, M.; Liu, D.; Hou, R.; Chen, D.; Wang, J. Abnormal Anionic Porphyrin Sensing Effect for HER2 Gene Related DNA Detection via Impedance Difference between MWCNTs and Single-Stranded DNA or Double-Stranded DNA. Molecules 2018, 23, 2688. [Google Scholar] [CrossRef] [Green Version]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and Sensitive Determination of Vanillin Based on a Glassy Carbon Electrode Modified with Cu2O-Electrochemically Reduced Graphene Oxide Nanocomposite Film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef] [Green Version]
- Koudelkova, Z.; Syrovy, T.; Ambrozova, P.; Moravec, Z.; Kubac, L.; Hynek, D.; Richtera, L.; Adam, V. Determination of Zinc, Cadmium, Lead, Copper and Silver Using a Carbon Paste Electrode and a Screen Printed Electrode Modified with Chromium(III) Oxide. Sensors 2017, 17, 1832. [Google Scholar] [CrossRef]
- Lakshmi, D.; Sharma, P.S.; Prasad, B.B. Imprinted polymer-modified hanging mercury drop electrode for differential pulse cathodic stripping voltammetric analysis of creatine. Biosens. Bioelectron. 2007, 22, 3302–3308. [Google Scholar] [CrossRef]
- Dinçkaya, E.; Sezgintürk, M.K.; Akyılmaz, E.; Ertaş, F.N. Sulfite determination using sulfite oxidase biosensor based glassy carbon electrode coated with thin mercury film. Food Chem. 2007, 101, 1540–1544. [Google Scholar] [CrossRef]
- Arduini, F.; Calvo, J.Q.; Palleschi, G.; Moscone, D.; Amine, A. Bismuth-modified electrodes for lead detection. TrAC Trends Anal. Chem. 2010, 29, 1295–1304. [Google Scholar] [CrossRef]
- Guzsvány, V.; Nakajima, H.; Soh, N.; Nakano, K.; Švancara, I.; Vytřas, K.; Bjelica, L.; Imato, T. Anodic Stripping Voltammetry Combined with Sequential Injection Analysis for Measurements of Trace Metal Ions with Bismuth- and Antimony Film Electrodes under Comparable Conditions. Electroanalysis 2011, 23, 1593–1601. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A. Stripping Analysis at Bismuth-Based Electrodes. Curr. Anal. Chem. 2008, 4, 183–190. [Google Scholar] [CrossRef]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef]
- Economou, A. Bismuth-film electrodes: Recent developments and potentialities for electroanalysis. TrAC Trends Anal. Chem. 2005, 24, 334–340. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, L.; Xing, S.; Xian, Y.; Shi, G.; Jin, L. Ultrasensitive voltammetric detection of trace lead (II) and cadmium (II) using MWCNTs-nafion/bismuth composite electrodes. Electroanal. Int. J. Devot. Fundam. Pract. Asp. Electroanal. 2008, 20, 2655–2662. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Miao, L.Q.; Zhang, Y.Y.; Cheng, S.M.; Sathishkumar, P.; Saravanakumar, B.; Nan, J.M.; Gu, F.L. 3D-Flower-Like Copper Sulfide Nanoflake-Decorated Carbon Nanofragments-Modified Glassy Carbon Electrodes for Simultaneous Electrocatalytic Sensing of Co-existing Hydroquinone and Catechol. Sensors 2019, 19, 2289. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M.; Coma, V. Preparation and characterization of active emulsified films based on chitosan-carboxymethyl cellulose containing zinc oxide nano particles. Int. J. Biol. Macromol. 2017, 99, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Wang, X.; Sun, Z.; Ma, J.; Wu, T.; Xing, F.; Gao, J. Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr. Polym. 2014, 101, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Manzoor, K.; Ahmad, S.; Ikram, S. Preparation, Kinetics, Thermodynamics, and Mechanism Evaluation of Thiosemicarbazide Modified Green Carboxymethyl Cellulose as an Efficient Cu (II) Adsorbent. J. Chem. Eng. Data 2018, 63, 1905–1916. [Google Scholar] [CrossRef]
- Yang, S.; Fu, S.; Liu, H.; Zhou, Y.; Li, X. Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Pejovnik, S.; Dominko, R.; Bele, M.; Gaberscek, M.; Jamnik, J. Electrochemical binding and wiring in battery materials. J. Power Sources 2008, 184, 593–597. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Thinakaran, N. Ultra sensitive detection of Cd (II) using reduced graphene oxide/carboxymethyl cellulose/glutathione modified electrode. Carbohydr. Polym. 2018, 197, 366–374. [Google Scholar] [CrossRef]
- Afkhami, A.; Bagheri, H.; Khoshsafar, H.; Saber-Tehrani, M.; Tabatabaee, M.; Shirzadmehr, A. Simultaneous trace-levels determination of Hg (II) and Pb (II) ions in various samples using a modified carbon paste electrode based on multi-walled carbon nanotubes and a new synthesized Schiff base. Anal. Chim. Acta 2012, 746, 98–106. [Google Scholar] [CrossRef]
- Janegitz, B.C.; Figueiredo-Filho, L.C.S.; Marcolino-Junior, L.H.; Souza Silvéria, P.N.; Pereira-Filho, E.R.; Fatibello-Filho, O. Development of a carbon nanotubes paste electrode modified with crosslinked chitosan for cadmium(II) and mercury(II) determination. J. Electroanal. Chem. 2011, 660, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Anandhakumar, S.; Mathiyarasu, J.; Phani, K.L. Anodic stripping voltammetric determination of cadmium using a “mercury free” indium film electrode. Analyst 2013, 138, 5674–5678. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Y.; Li, Q.; Chen, X.; Xu, X. Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int. J. Biol. Macromol. 2019, 126, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hu, Z. Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J. Electroanal. Chem. 2005, 583, 46–55. [Google Scholar] [CrossRef]
- Maghear, A.; Tertis, M.; Fritea, L.; Marian, I.O.; Indrea, E.; Walcarius, A.; Sandulescu, R. Tetrabutylammonium-modified clay film electrodes: Characterization and application to the detection of metal ions. Talanta 2014, 125, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, B.; Ji, L.; Wang, F.; Yuan, Q.; Hu, G.; Dong, A.; Gan, W. An efficient electrochemical sensor based on three-dimensionally interconnected mesoporous graphene framework for simultaneous determination of Cd(II) and Pb(II). Electrochim. Acta 2016, 222, 1371–1377. [Google Scholar] [CrossRef]
- Thinakaran, N.; Subramani, S.E.; Priya, T.; Dhanalakshmi, N.; Vineesh, T.V.; Kathikeyan, V. Electrochemical Determination of Cd2+ and Pb2+ Using NSAID-mefenamic Acid Functionalized Mesoporous Carbon Microspheres Modified Glassy Carbon Electrode. Electroanalysis 2017, 29, 1903–1910. [Google Scholar] [CrossRef]
- Shah, A.; Sultan, S.; Zahid, A.; Aftab, S.; Nisar, J.; Nayab, S.; Qureshi, R.; Khan, G.S.; Hussain, H.; Ozkan, S.A. Highly sensitive and selective electrochemical sensor for the trace level detection of mercury and cadmium. Electrochim. Acta 2017, 258, 1397–1403. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Zhang, L.; Xing, R.; Jiao, T.; Gao, F.; Peng, Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd (II) ions. Nanotechnology 2018, 29, 445603. [Google Scholar] [CrossRef]
- Jiang, Q.-M.; Zhang, M.-R.; Hou, F.; Wang, Z.-G.; Zhang, S.-H.; Chen, Y.; Luo, L.-Q.; Pan, G.-B. In situ bismuth-modified gallium nitride electrode for sensitive determination of cadmium (II) with high repeatability. J. Electroanal. Chem. 2018, 809, 105–110. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Shamsipur, M. Modification of gold surface by electrosynthesized mono aza crown ether substituted catechol-terminated alkane dithiol and its application as a new electrochemical sensor for trace detection of cadmium ions. Colloids Surf. B Biointerfaces 2018, 171, 494–500. [Google Scholar] [CrossRef]
- Qin, D.; Wang, L.; Gao, S.; Wang, Y.; Mamat, X.; Li, Y.; Wagberg, T.; Cheng, H.; Hu, G. N-Doped Hollow Porous Carbon Spheres/Bismuth Hybrid Film Modified Electrodes for Sensitive Voltammetric Determination of Trace Cadmium. Electroanalysis 2018, 30, 1906–1912. [Google Scholar] [CrossRef]
- Wu, X.; Wu, S.; Li, Y.; Chen, H.; Yuan, Q.; Gan, W. A highly sensitive electrochemical sensor for Cd (II) based on protic salt derived nitrogen and sulfur co-doped porous carbon. Anal. Chim. Acta 2019, 1046, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Tothill, I.E. Ressolving the copper interference effect on the stripping chronopotentiometric response of lead(II) obtained at bismuth film screen-printed electrode. Talanta 2005, 66, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.; Cheng, J. Simultaneous determination of trace Cd (II) and Pb (II) based on Bi/Nafion/reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition. Ionics 2016, 23, 767–777. [Google Scholar] [CrossRef]













| Modified Electrodes | Methods | Linear Range (μmol/L) | LOD (μmol/L) | Ref. |
|---|---|---|---|---|
| GCE+MMT+TBAB (partial)/M | SWASV a | 0.2–2.5 | 0.072 | [44] |
| Bi-MGF/GCE | DPASV b | 0.017–0.625 | 0.004 | [45] |
| MC/MA/Nafion/GC | SWASV | 0.05–0.3 | 0.024 | [46] |
| PPE-GCE | SWASV | 0.017–0.89 | 0.007 | [47] |
| CNT-SO3H/RhB-LB/GCE | SWASV | 0.1–1.2 | 0.08 | [48] |
| Bi/GaN | SWASV | 0.009–1.334 | 0.0027 | [49] |
| SAM electrode | DPV c | 15–65 | 4.5 | [50] |
| Bi-N-HPCSs/GCE | DPASV | 0.004–1.33 | 0.001 | [51] |
| N,S-PC-Nafion/Bi/GCE | DPASV | 0.035–0.714 | 0.0008 | [52] |
| Bi/CMC-GCE | DPASV | 0.001–1 | 0.00075 | This work |
| Sample | Added (μmol/L) | Found (μmol/L) | Recovery (%) |
|---|---|---|---|
| Tap water | - | - | - |
| 0.01 | 0.011 | 110 | |
| 0.02 | 0.021 | 105 | |
| 0.10 | 0.09 | 93.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Luo, X.; Wang, F.; Huang, S.; Wang, J.; Liu, D.; Liu, D.; Chen, D.; Wei, J.; Liu, Y. Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry. Sensors 2019, 19, 5482. https://doi.org/10.3390/s19245482
Ning J, Luo X, Wang F, Huang S, Wang J, Liu D, Liu D, Chen D, Wei J, Liu Y. Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry. Sensors. 2019; 19(24):5482. https://doi.org/10.3390/s19245482
Chicago/Turabian StyleNing, Jingheng, Xin Luo, Faxiang Wang, Shouen Huang, Jianhui Wang, Dongmin Liu, Donglin Liu, Donger Chen, Jiaqian Wei, and Yongle Liu. 2019. "Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry" Sensors 19, no. 24: 5482. https://doi.org/10.3390/s19245482
APA StyleNing, J., Luo, X., Wang, F., Huang, S., Wang, J., Liu, D., Liu, D., Chen, D., Wei, J., & Liu, Y. (2019). Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry. Sensors, 19(24), 5482. https://doi.org/10.3390/s19245482